Abstract
Introduction
Multicenter, randomized, double-blinded crossover study. The Netherlands (ClinicalTrials.gov NCT02112474). We hypothesized that the pain suppressive effects of 1000 Hz and 30 Hz spinal cord stimulation (SCS) strategies are equally effective in patients with chronic, neuropathic, unilateral leg pain after back surgery.
Methods
Thirty-two patients (18–70 years, minimum leg pain 50 mm on 100 mm visual analog scale (VAS), minimal back pain) were randomized (1:1) to start 1000 Hz or 30 Hz neurostimulation for 9 days. After a 5-day washout, they crossed over, for another 9 days. Primary outcome was pain suppression (mean of VAS scores 4×/day) during the crossover period. The main investigators were blinded to strategy allocation, patients were blinded to the outcome, a blinded assessor analyzed the primary outcome.
Results
The primary outcome was analyzed in 26 patients. There was no period effect (delta 4 mm, p = 0.42, 95% CI [− 5, 13]), allowing direct intrapatient comparison of the treatment effect (delta 1 mm, p = 0.92, 95% CI [− 13, 14]). Ninety-two percent of patients in both periods experienced greater than 34% pain suppression (minimal clinically important difference, MCID). Secondary outcomes (22 patients): pain suppression and improved quality of life were sustained at 12 months; both were statistically significant and clinically relevant. Fifty percent of patients had greater than 80% pain suppression (p < 0.001).
At study termination, all events were resolved; no unanticipated events were reported. Medtronic provided a grant for additional study costs.
Conclusion
We conclude that our hypothesis regarding the effect of 1000 Hz and 30 Hz stimulation strategies on pain suppression was confirmed. Both stimulation strategies led to a large, sustainable, clinically relevant pain suppression and improvement in quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Multicenter, randomized, double-blinded crossover study. | |
Homogenous patient group: chronic, unilateral, neuropathic leg pain after back surgery. | |
Comparison of 1000 Hz and 30 Hz in the 30-day trial period. | |
There were no significant differences in the treatment effects of 1000 Hz and 30 Hz at the end of the trial period. | |
A majority of the patients (16/27) preferred sub-perception threshold stimulation but this was not based on their pain suppression. | |
Ninety-two percent of patients experienced a minimally important clinical difference (greater than 34% pain suppression). | |
Fifty percent of patients experienced greater than 80% pain suppression at 1 year. | |
Quality of life according to patient-reported outcome measures improved at 1 month and were sustained through 1 year. |
Digital Features
This article is published with digital features, including a summary slide to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14447637.
Introduction
Chronic pain is a widespread disease, of moderate to severe intensity, which occurs in approximately 19% of adult Europeans, seriously affecting the quality of their social and working lives [1]. Chronic pain can be classified into six categories, including chronic neuropathic pain, according to the new International Classification of Diseases, version 11 (ICD-11) [2]. The effectiveness of conservative medical management is limited and treatments such as neurostimulation are used. One specific group with chronic neuropathic pain is patients with failed back surgery syndrome (FBSS). Spinal cord stimulation (SCS) is well established in the treatment of the neuropathic pain components of FBSS [3].
Conventional SCS stimulation (30–80 Hz) is a treatment option for patients with FBSS. At the conception of this study, a number of alternative SCS strategies became available. These strategies utilize a higher electrical frequency than conventional SCS (up to 10 kHz). Randomized controlled trials (RCTs) using both conventional and higher frequencies showed a difference in effect between back and leg pain, and between conventional and higher frequencies [4,5,6,7].
The pathophysiology of low back pain is complex and includes mechanical disorders, non-mechanical diseases, and organ diseases. Low back pain may therefore consist of a mechanical, nociceptive, and/or neuropathic component, of which only neuropathic pain responds to spinal cord stimulation [8]. We therefore chose to perform a study in the most well accepted and clearly defined patient group for SCS, namely predominant (greater than 80%) unilateral neuropathic leg pain. In order to evaluate if one of these stimulation strategies would be an equally effective and advantageous treatment for unilateral neuropathic leg pain in stimulation-naïve patients, we decided to perform a study comparing a 1000 Hz strategy (the maximum frequency available in this neurostimulation system) to a conventional SCS strategy (30 Hz).
We hypothesized that SCS is effective in the treatment of unilateral neuropathic leg pain, and that a 1000 Hz strategy is equally as effective as a 30 Hz strategy in pain suppression.
Methods
Design and Setting
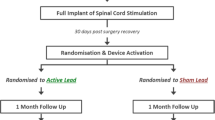
This multicenter, double-blind, randomized crossover study compared pain suppression in a 1000 Hz versus 30 Hz strategy (Fig. 1) in patients with unilateral neuropathic leg pain during the SCS trial. The study was approved by the Medische Ethische Toetsings Commissie (METC) of the Amsterdam UMC, location AMC, on 23 December 2013 (METC 2013_271) and registered with ClinicalTrials.gov (NCT02112474) [9] on 25 March 2014, before the first patient was enrolled (13 November 2014). It was conducted in compliance with all applicable regulatory and institutional guidelines (Declaration of Helsinki 1964 and its later amendments). The Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines and extension for crossover trials [10] and International Committee of Medical Journal Editors (ICMJE) recommendations [11] for reporting were used in the writing of this report.
Participants
Stimulation-naïve patients from five large pain centers in the Netherlands, experienced in neurostimulation, were approached to take part in the study. These patients were already selected for neurostimulation according to national standards (all had received conservative medical management and minimally invasive treatments with insufficient effect or unacceptable side effects).
Inclusion criteria were age 18–70 years, unilateral neuropathic leg pain (dermatomes L4/L5/S1), a minimum visual analogue scale (VAS) leg pain score of at least 50 mm on a 100 mm scale, with minimal back pain (VAS less than 40 mm) following back surgery, for a minimum of 6 months, and stable use of medication in the preceding 2 months.
Study Procedures
All patients signed an ethical committee approved informed consent before taking part in the study and for the use of their data in publications. Baseline parameters were collected (demographic data, short medical history, types of pain medication used, current pain), and the patients completed several validated questionnaires on patient-reported outcome measures (PROMs).
Patients were implanted with one or two 8-polar leads, which were connected to an external neurostimulator (Restore Sensor™, made available for study purposes by Medtronic International Trading Sàrl, Tolochenaz, Switzerland), to facilitate both programming strategies. If both strategies could be programmed with the first lead, only one lead was placed. If the 30 Hz strategy stimulation gave less than 80% coverage of the primary pain area, a second lead was placed.
Stimulation Strategies
Stimulation strategies and electrode settings were standard of care in the study centers.
Strategy 1000 Hz: 9 days, bipolar electrode setting over T9–T10, 220 µs (maximum possible pulse width related to frequency), amplitude 20% below perception level in the supine position. Stimulation was not perceptible.
Washout: A minimum of 5 days, all stimulation turned off (programming removed from stimulator).
Strategy 30 Hz: 9 days, three or four electrode setting (with anode(s) between cathodes) placed where the best conventional stimulation was felt, 300 µs (with the option of optimizing up to 450 µs), amplitude individualized to supra-perception and maximum comfort. Stimulation was perceptible.
After lead implantation, patients were randomized to one of the two strategies for 9 days (period 1). After this, they underwent a 5-day washout period, with no stimulation, followed by a crossover to the other strategy in period 2.
Apart from existing (stable) medication, no concurrent treatment was allowed during the crossover period.
Patients experiencing a minimum of 50% pain suppression, in either period, received an internal pulse generator (IPG), at the end of period 2. At the end of the trial period (after the second stimulation period), all patients indicated their preference for stimulation strategy. In the open-label period (12 months), patients were free to change stimulation strategies.
Primary Outcome
The primary outcome of the study was the comparison in amount of pain suppression, in the crossover period, between 1000 and 30 Hz stimulation strategy during the SCS trial.
Secondary Outcomes
Secondary outcomes included pain suppression, PROMs on quality of life (EQ-5D-3L and SF-36), sleep, patient satisfaction, employment status, and global perceived effect scale (GPEs) of change at 12 months follow-up. We also collected patient preference at the end of the crossover design and reviewed the reported adverse events.
Measurements
Pain measurements were collected on self-reported paper pain diaries with VAS (100 mm line, anchored with “no pain” and “maximum pain”), scored four times a day (3 day measurements and one night measurement on awakening). The mean of all scores was calculated.
The validated PROMs were completed during the regular visits to the outpatient clinic, before optimization of the therapy. Quality of life was measured with EQ-5D-3L (EuroQol group) and SF-36™, v2.0 (RAND Corporation). In addition, we evaluated sleep and patient satisfaction, using Likert items at baseline, 1 month, and 12 months. The Global Perceived Effect scale was used to evaluate the patients’ recovery of complaints at 1 month and 12 months.
Serious and Adverse Events
We collected all (serious) adverse events related to the device or stimulation strategy, actively at each follow-up visit or spontaneously reported.
Sample Size
A 20-mm difference in the delta change of VAS scores between the results of 1000 Hz compared to 30 Hz stimulation strategy was expected. Sample size calculation using G*Power [8, 12], indicated that a minimum of 17 patients were required to answer the research question (H0: pain suppression 1000 Hz = pain suppression 30 Hz), with an α set at 0.05 and β at 0.20. To account for non-responders (expected trial-to-implantation rate of 90%) and attrition due to withdrawals or lost to follow-up, we increased the sample size to 30 and no patients were replaced.
Allocation Concealment and Blinding
After lead placement and before the start of the crossover period, the site sent an e-mail to an independent third party (AW), who used the ALEA computerized system to generate the allocation for the patient. Allocation to one of the stimulation strategies (1000 Hz or 30 Hz first) was performed on a 1:1 basis, using random blocks of four. The site received the allocation by e-mail.
JB and FW were blinded to stimulation allocation throughout the study (no access to the ALEA system) and did not treat any patients. All other investigators and treating staff were not blinded. After blinded analysis of the primary outcome (JB) and interpretation of the results (JB + FW), allocation was unblinded by an independent third party (AW). To prevent bias of pain reporting, patients were blinded to the true outcome of the study (pain suppression). Patients were given to understand that the primary outcome was their preference for the stimulation strategy (as explained in the patient information).
Data Management and Statistics
Data were monitored by an independent party, according to a pre-defined plan. No interim analysis was performed. A GCP-compliant database (Castor EDC, version 2019.1.5) [13], was used for electronic data entry. A random 10% sample of the entered data was double-checked. Queries were resolved before the database was locked. Data analysis was performed with SPSS (version 24.0) and R (version 3.5.1 (2 July 2018)—“Feather Spray”).
Patient characteristics are presented with descriptive statistics as a mean with standard deviation, or a median with interquartile range for continuous data and absolute numbers with a percentage for categorical data.
Primary outcome data were screened for outliers and missing data, and no data were imputed. All patients who had a complete dataset for baseline and both trial periods were analyzed. The period effect was defined as the difference between the delta VAS scores of baseline–period 1 (T0–T1) and baseline–period 2 (T0–T2). The treatment effect was defined as the difference between the mean delta VAS scores of 1000 Hz and 30 Hz. The period and treatment effects were calculated using the two-sided Wilcoxon rank sum test (Mann–Whitney U) for non-normally distributed continuous data, with Hodges–Lehmann estimator for the 95% confidence interval. A p value of less than 0.05 was considered statistically significant.
Secondary outcome data only included patients who were implanted with a neurostimulator and had a 12-month follow-up. Descriptive and inferential statistics were used as appropriate. Outcomes were reported using either parametric or non-parametric testing, depending on the distribution. Pain scores were tested for significance with a Friedman’s test. Effect size was calculated with Kendall’s W. Post hoc testing was done with a Wilcoxon signed rank sum and Bonferroni correction. Quality of life was analyzed according to the specific guidelines by EQ-5D-3L and SF-36. The EQ-5D-3L index scores were calculated according to the Dutch time trade-off (TTO) population validation. Kruskal–Wallis and Dunn’s test with Holm correction were used for statistical testing. The SF-36™, v2.0 scores were calculated according to the scoring system of the RAND SF-36. Data were tested with a repeated-measures analysis of variance (ANOVA) followed by post hoc testing with a Bonferroni correction.
Results
Thirty-two patients signed informed consent and were enrolled and randomized. Two patients had to be excluded after randomization as they retrospectively did not meet the inclusion criteria. The study was completed according to protocol with 30 patients. Data were collected from November 2014 to October 2018 (12 month follow-up of last patient). Twenty-six of the 30 randomized patients available had a complete set of data needed for the analysis of the primary outcome; four patients were removed from this analysis beause of incomplete data (Fig. 2).
The baseline characteristics of the 26 patients included in the primary analysis are described in Table 1. The mean VAS at baseline was 68 mm (± 12 mm). The median duration of chronic pain was 8 years (range 1–36 years). Previous medical history showed that 81% of patients had used opioids (50% used oxycodone), 54% had used non-steroidal anti-inflammatory drugs (NSAIDs), most notably diclofenac (56%), and 73% had used antineuropathics [primarily pregabaline (57%)]. Seven patients had used three or more medication groups simultaneously. All patients underwent one or more nerve root blocks and seven patients had undergone an epiduroscopy.
Primary Outcome
Pain Scores in Crossover Period
Figure 3a represents the VAS per period (baseline, period 1, washout, period 2). This was calculated as a mean value over the specific period and based on the multiple entries per day in the weighted VAS diaries.
a Mean VAS during the crossover period. VAS visual analog scale. Mean VAS during crossover period. There was no statistically significant difference between period 1 and 2 (no period effect), enabling the periods to be combined for analysis of the treatment effect. There was no statistical difference in the pain suppression effect of 1000 Hz and 30 Hz. b Percentage pain suppression in open-label follow-up at 12 months. MCID minimal clinically important difference, VAS visual analog scale. Percentage pain suppression in open-label follow-up at 12 months: 85% of the patients had greater than 34% pain suppression (MCID), 80% of the patients had greater than 50% pain suppression, and 45% patients had greater than 80% pain suppression
Period Effect and Treatment Effect
In a crossover design, the period effect is first tested. If there is no statistically significant difference, both periods can be pooled to determine the treatment effect. The period effect was not statistically significant (delta 4 mm, p = 0.42, 95% CI [− 5, 13]). The results of both periods could therefore be pooled, allowing direct intrapatient comparison for the treatment effect of 1000 Hz and 30 Hz strategies. There was no statistically significant difference in the pain suppression of 1000 Hz in comparison with 30 Hz strategy (delta 1 mm, 95% CI [− 13, 14], p = 0.92). The null hypothesis was not rejected.
Percentage of Pain Suppression
In period 1, 69% of patients achieved greater than 50% pain suppression and 92% of patients achieved at least 34% pain suppression, representing an MCID from a patient’s perspective [14]. The results in period 2 were similar.
Patient Preference for Stimulation
At the end of the crossover period, the majority (16/27) reported a preference for the 1000 Hz strategy; however, this was not statistically significant.
Secondary Outcomes
Of the 32 patients enrolled, 27 received a neurostimulator (trial-to-implant ratio of 84%) and 22 patients were followed for 12 months, and used for analysis of the secondary outcomes (Fig. 3b). The attrition rate (implanted patients lost to follow-up, 5/27) was 19%. Additional columns were added to Table 1, to describe the characteristics of the 22 analyzed at 12 months and the five who were lost to follow-up.
Pain Suppression at 12 Months
VAS scores of complete sets (n = 16) were compared between baseline, 1 month, and 12 months. Three patients were missing the 1-month scores. Mean VAS at baseline was 64 mm (± 15 mm), at 1 month 16 mm (± 20 mm), and at 12 months 19 mm (± 18 mm). The VAS score was statistically significantly different over time (Friedman test, χ2 = 18.97, p < 0.001).
A pairwise Wilcoxon signed rank test between groups revealed statistically significant differences in VAS score between baseline and 1 month (p adj. < 0.01) and baseline and 12 months (p adj. < 0.01); there was no statistically significant difference between 1 and 12 months (p adj. = 1.00).
At 1 month, 94% of the patients experienced greater than 34% pain suppression and 56% had greater than 80% pain suppression. At 12 months, 84% of the patients experienced greater than 34% pain suppression and 47% of the patients experienced greater than 80% pain suppression (Fig. 3b).
EQ-5D-3L
A comparison was made over time using a Kruskal–Wallis test (p < 0.01). Post hoc testing (between baseline–1 month, baseline–12 months, and 1 month–12 months) was done with a Dunn’s test and Holm correction. There is a statistically significant improvement in the index scores from baseline to 1 month (p < 0.001) and baseline to 12 months (p < 0.01). There is no difference in the index scores between 1 and 12 months (p = 0.60). Index scores rose from a median of 0.334 to 0.811 and 0.809, at 1 month and 12 months, respectively. A clinically relevant result was also seen here; the normal value for the Netherlands is 0.843 (perfect health with moderate pain/discomfort) (Fig. 4a).
a Quality of life—EQ-5D-3L. EQ-5D-3L: Baseline, 1 month, and 12 months scores calculated from Dutch time trade-off norms. A normal value for the Netherlands is 0.843 (perfect health with moderate pain/discomfort). *Indicates a statistically significant difference between baseline and 1 month (p < 0.001) and ** a statistically significant difference between baseline and 12 months (p < 0.01). b Quality of Life—SF-36. PF physical functioning, RP role physical, RE role emotional, VT energy/vitality, MH mental health, SF social functioning, BP bodily pain, GH general health. There was a statistically significant improvement between baseline and 1 month, and baseline and 12 months, in all domains of the SF-36, with the exception of mental health and general health
SF-36
Complete data was available for 22 patients. The RM-ANOVA score was statistically significantly different over the time period [F(2,16) = 19.0, p < 0.001, generalized eta squared = 0.27]. Post hoc analyses with a Bonferroni adjustment revealed that two pairwise comparisons (baseline–1 month and baseline–12 months) were statistically significant (p < 0.01). The improvement at 1 month was sustained at 12 months (Fig. 4b).
Sleep
Patients were asked about the quality of sleep in the week before the hospital visit. The number of patients reporting an improvement in quality of sleep doubled from baseline (six patients) to 1 month (12 patients) and remained steady at 12 months. Eight patients reported no change.
Patients were also asked to compare their quality of sleep before and after SCS. At 1 month, 75% of patients reported an improvement; at 12 months, 77% reported improvement.
Employment
The employment status at baseline was unknown for two patients, three were pensioners, six were working (three full-time), and 11 were on disability grant/sick leave. At 12 months, the status for two patients was still unknown, three were pensioners, ten patients were working (four full-time), and seven were on disability grant/sick leave.
Satisfaction and GPEs
At 1 month, 91–100% of the patients were satisfied with pain suppression, with the therapy, and with the change in pain as a result of SCS and would undergo SCS again. At 12 months the satisfaction in the aforementioned areas ranged from 77% to 91%.
On the GPEs, “some to complete recovery” was reported by 100% of the patients at 1 month and 91% at 12 months. No patients reported a “worsening of complaints” at any time point.
Serious Adverse Events
Only device- and stimulation-related adverse events in the period between implantation and 12 months follow-up were recorded and reported. No unanticipated events were reported and all events were resolved at the end of the study. Only one serious adverse event was reported in one patient (soft tissue infection followed by explantation of the system). There were 13 reported adverse events in eight patients. One adverse event was an increase in pain at 12 months (resolved by reprogramming of the neurostimulator). Six lead migrations occurred, five during the trial period and one during follow-up. There were six pocket problems reported, four were resolved operatively, two resolved spontaneously.
Discussion
We found that in our crossover study utilizing 1000 Hz and 30 Hz (each patient received both strategies), both strategies have proven to be equally effective in pain suppression in the SCS trial. In the open-label period (12 month follow-up), a large, sustainable, and clinically relevant pain suppression and improvement in quality of life was seen.
Different SCS strategies have proven to be effective for pain suppression in populations with mixed back and leg pain. The majority of these studies included patients with a larger back pain component, patients who had already received stimulation before the study, or who failed conventional SCS. Although some of these studies report on leg pain, this is only part of a subanalysis and not a primary outcome [7, 15,16,17,18]. Our study design differed in the following points: we had a homogenous group with unilateral neuropathic pain, a minimal back pain component, suppression of leg pain was the primary outcome, no active stimulation in the washout period, and the use of 1000 Hz and 30 Hz. We showed that our strategies had a large effect on the pain suppression in highly selected patients with leg pain.
The profound, clinically relevant pain suppression that we found at 1 month was sustained at 12 months. Commonly reported cutoff values of pain suppression are 34% (MCID), 50%, and 80%. We found that 84%, 79%, and 47% of our patients, respectively, were above these values at 12 months. The pain suppression in our study falls within the specific leg pain ranges published in larger RCTs, which compared conventional SCS with different alternative strategies [7, 15]. Kapural et al. reported 51–78% of patients with greater than 50% and 42–67% of patients with greater than 80% leg pain suppression. The Evoke study, by Mekhail et al., reported 61–83% of patients with greater than 50% and 37–56% of patients with greater than 80% leg pain suppression.
The EQ-5D-3L improved at 1 month and was sustained through 12 months, at a level which is almost equivalent to the Dutch National norm, 0.843 (perfect health with moderate pain/discomfort). The SF-36 also showed statistically and clinically relevant improvements. Patients were more socially active and reported better general health, approaching the National Dutch SF-36 norms for the majority of the domains [19]. This is in line with other publications on SCS, reporting an improvement in quality of life [4, 16]. Little is known about the improvement in employment status of SCS patients in the long term. In an RCT comparing SCS to conventional medical management, those who received SCS improved their employment status [20]. In our study, the improvement in SF-36 domains reflecting employment status, in combination with pain suppression, might be related to the positive changes in quality of life.
Sleep is seldom used as an outcome measure, although patients with chronic pain often report sleep disturbances as a factor affecting their well-being and pain. A recent systematic review revealed that sleep is only used as a secondary outcome in 20.6% of the included SCS studies [21]. Following initiation of SCS therapy, we saw an improvement in different aspects of sleep, reported by 75–77% of patients. In addition, the majority of patients were very satisfied with the SCS therapy and more than half of the patients reported the effect of the SCS therapy as “almost complete recovery of complaints” or “complete recovery of complaints”.
Several papers have looked at possible factors that influence patient preference for a specific stimulation strategy [3, 22,23,24]. The choice appears to be highly individual and possibly related to age, technical ability, activity, or previous experience with paresthesias. Patients choosing paresthesia-based strategies do so for reasons of control over the therapy, a preference for the “tingling” sensation or a lack of maintenance (recharging burden). Although pain suppression plays a role, it would not appear to be the deciding factor. In our study, where pain suppression with both strategies was equal, the majority of these stimulation-naïve patients preferred the 1000 Hz, although this was not statistically significant.
Our study is unique in that it evaluated a well-defined, homogeneous, stimulation-naïve group of patients with unilateral neuropathic leg pain in a double-blind crossover design. Patients, main investigators, and assessors were blinded. The study was sufficiently powered to produce a robust effect size. Despite the length of time needed to include patients, we were able to complete enrollment according to the a priori sample size calculation. The trial to implant ratio (84%) fell within the ranges reported in other RCTs (75–94%) [4,5,6, 15]. Furthermore, our attrition rate (19%) falls within the accepted quality requirements for neurostimulation studies (less than 20%) [25].
A limitation of the study is that we did not ask patients the reason for their preference for stimulation. Furthermore, the generalizability of the results of this study may be limited by the low number of patients with FBSS and predominant unilateral neuropathic leg pain. The majority of them either had a larger back pain component and/or radiation to both legs. We found that the pain scores in the washout period for both strategies did not return to baseline. We do not think this influenced our results, as the possible bias is minimized by the crossover design.
We conclude that a 1000 Hz strategy is equally effective for pain suppression as a 30 Hz strategy, in this population of patients with FBSS with unilateral neuropathic leg pain, at the end of the crossover period.
In the long term, pain suppression and quality of life showed statistically and clinically relevant improvements at 1 month that are sustained through to 12 months. Patients also reported an improvement in sleep and satisfaction with the therapy.
On the basis of the large effect size found, we would recommend further comparative studies in well-defined patient groups and indications, evaluating the optimal SCS strategy for chronic pain, with more focus on factors that influence patient preference.
References
Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. https://doi.org/10.1016/j.ejpain.2005.06.009.
Barke A. Chronic pain has arrived in the ICD-11. IASP. 2019. https://www.iasp-pain.org/PublicationsNews/NewsDetail.aspx?ItemNumber=8340. Accessed 23 Mar 2021, Verified 13 May 2021.
Head J, Mazza J, Sabourin V, et al. Waves of pain relief: a systematic review of clinical trials in spinal cord stimulation waveforms for the treatment of chronic neuropathic low back and leg pain. World Neurosurg. 2019;131(264–74):e3. https://doi.org/10.1016/j.wneu.2019.07.167.
Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79(5):667–77. https://doi.org/10.1227/neu.0000000000001418.
De Andres J, Monsalve-Dolz V, Fabregat-Cid G, et al. Prospective, randomized blind effect-on-outcome study of conventional vs high-frequency spinal cord stimulation in patients with pain and disability due to failed back surgery syndrome. Pain Med. 2017;18(12):2401–21. https://doi.org/10.1093/pm/pnx241.
Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21(1):56–66. https://doi.org/10.1111/ner.12698.
Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 Therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851–60. https://doi.org/10.1097/aln.0000000000000774.
Rigoard P, Delmotte A, D’Houtaud S, et al. Back pain: a real target for spinal cord stimulation? Neurosurgery. 2012;70(3):574–84. https://doi.org/10.1227/NEU.0b013e318236a57c (Discussion 84–5).
ClinicalTrials.gov. NCT02112474. 2014. https://www.clinicaltrials.gov/ct2/show/NCT02112474?term=NCT02112474&draw=2&rank=1.
Consolidated Standards of Reporting Trials (CONSORT). www.consort-statement.orghttp://www.consort-statement.org/. Accessed 23 Mar 2021.
International Committee of Medical Journal Editors (ICMJE). www.icmje.org.
Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. https://doi.org/10.3758/bf03193146.
Castor EDC. Castor Electronic Data Capture 2019 [27 Aug. 2019]. https://castoredc.com. Accessed 28 Aug 2019.
Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21. https://doi.org/10.1016/j.jpain.2007.09.005.
Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123–34. https://doi.org/10.1016/s1474-4422(19)30414-4.
Manca A, Kumar K, Taylor RS, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain. 2008;12(8):1047–58. https://doi.org/10.1016/j.ejpain.2008.01.014.
Perruchoud C, Eldabe S, Batterham AM, et al. Analgesic efficacy of high-frequency spinal cord stimulation: a randomized double-blind placebo-controlled study. Neuromodulation. 2013;16(4):363–9. https://doi.org/10.1111/ner (Discussion 69).
Thomson SJ, Tavakkolizadeh M, Love-Jones S, et al. Effects of rate on analgesia in kilohertz frequency spinal cord stimulation: results of the PROCO randomized controlled trial. Neuromodulation. 2018;21(1):67–76. https://doi.org/10.1111/ner.12746.
Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055–68. https://doi.org/10.1016/s0895-4356(98)00097-3.
Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63(4):762–70. https://doi.org/10.1227/01.Neu.0000325731.46702.D9 (Discussion 70).
McNicol E, Ferguson M, Bungay K, et al. Systematic review of research methods and reporting quality of randomized clinical trials of spinal cord stimulation for pain. J Pain. 2020. https://doi.org/10.1016/j.jpain.2020.05.001.
Duse G, Reverberi C, Dario A. Effects of multiple waveforms on patient preferences and clinical outcomes in patients treated with spinal cord stimulation for leg and/or back pain. Neuromodulation. 2019;22(2):200–7. https://doi.org/10.1111/ner.12899.
Tjepkema-Cloostermans MC, de Vos CC, Wolters R, et al. Effect of burst stimulation evaluated in patients familiar with spinal cord stimulation. Neuromodulation. 2016;19(5):492–7. https://doi.org/10.1111/ner.12429.
Kriek N, Groeneweg JG, Stronks DL, et al. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double-blind, randomized and placebo-controlled crossover trial. Eur J Pain. 2017;21(3):507–19. https://doi.org/10.1002/ejp.944.
Taylor RS, Desai MJ, Rigoard P, et al. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysis. Pain Pract. 2014;14(6):489–505. https://doi.org/10.1111/papr.12095.
Acknowledgements
Patient follow-up and data collection: M. de Klerk-de Ruiter, M.R. Kruis, K.P. Hendrickx, M.M. de Roos, C. Terwiel, C. Edelbroek, P.S. van Daalen, M. Hemels-Kouwen, A.J.M. Kroon, H. Festen-van der Weijde, A. Dekkers, C. de Gooier- van Ekris, L. Ekelschot, J. Leroux, A.A.J. van der Voort, Data entry: B. Geraedts. Independent physician: O. Teernstra. Clinical Epidemiologist: S. van Dieren.
Funding
The study and journal’s Rapid Service Fee was funded by AMC Medical Research. The Amsterdam UMC, location AMC received a research grant from Medtronic. All participating sites received part of the equipment needed for the study free of charge (One RestoreSENSOR to be used as external stimulator, 1 × 8 Octad electrode sets and connection cables). Devices were reimbursed as in normal care. The financial support did not waive the rights of the participating sites to publish the results without undue delay. Medtronic did not have any role in the study design, data collection, analysis, or writing of the report. The Academic Medical Center received a small grant from Medtronic for data management costs and some equipment.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Jennifer Breel and Frank Wille designed the protocol, collected data, managed the study, conducted statistical analysis and drafted the manuscript. They both contributed equally and are co-first authors. Carin Wensing helped design the protocol, manage the study, performed randomization, and drafted the manuscript. Jan Willen Kallewaard, Katja Burger, Stijn de Graaf, Harmen Pelleboer and Xander Zuidema approved the protocol, implanted patients and approved the manuscript.
Disclosures
Jennifer Breel had a consultancy for speaking fees with Medtronic, Abbott and Saluda Medical. Frank Wille reports grants and non-financial support from Medtronic. Jan Willem Kallewaard is a member of the Advisory Boards of Medtronic, Saluda, Abbott, Nevro and Boston Scientific. Markus Hollmann, Carin Wensing, Xander Zuidema, Stijn de Graaf, Harmen Pelleboer, Katja Burger have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the Medische Ethische Toetsings Commissie (METC) of the Amsterdam UMC, location AMC, on 23 December 2013 (METC 2013_271) and registered with ClinicalTrials.gov (NCT02112474) [9] on 25 March 2014, before the first patient was enrolled (13 November 2014). It was conducted in compliance with all applicable regulatory and institutional guidelines (Declaration of Helsinki 1964 and its later amendments). The Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines and extension for crossover trials [10] and International Committee of Medical Journal Editors (ICMJE) recommendations [11] for reporting were used in the writing of this report. All patients signed an ethical committee approved informed consent before taking part in the study and for the use of their data in publications.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Data will be made available on request after an embargo period and with restricted access.
Conflicts of interest
Mrs J. Breel had consultancies with Medtronic and Abbott at the time of the study. Dr F. Wille had consultancies with Medtronic and Abbott at the time of the study. Ms. A. Wensing was an employee of Abbott at the time of the study. Dr J.W. Kallewaard was and is on the Advisory Boards of Medtronic, Saluda, Abbott, Nevro and Boston Scientific during the study and currently. Dr K. Burger, Dr X. Zuidema, Dr H. Pelleboer, Dr S. de Graaf and Professor M.W. Hollmann have no disclosures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jennifer Breel and Frank Wille are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Breel, J., Wille, F., Wensing, A.G.C.L. et al. A Comparison of 1000 Hz to 30 Hz Spinal Cord Stimulation Strategies in Patients with Unilateral Neuropathic Leg Pain Due to Failed Back Surgery Syndrome: A Multicenter, Randomized, Double-Blinded, Crossover Clinical Study (HALO). Pain Ther 10, 1189–1202 (2021). https://doi.org/10.1007/s40122-021-00268-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-021-00268-7