Abstract
Neuropathic pain develops when the somatosensory nervous system is affected by a lesion or disease. Diagnostic tests aimed at assessing somatosensory afferent pathway damage are therefore useful for diagnosing neuropathic pain. Neuropathic pain manifests with a range of different symptoms such as ongoing burning pain, squeezing or pressure pain, paroxysmal electric shock-like sensations, stabbing pain, or mechanical dynamic allodynia. The various types of neuropathic pain are associated with different underlying nerve fiber abnormalities. This article summarizes the available methods of somatosensory afferent pathway assessment and discusses the potential pathophysiology underlying the most representative neuropathic pain types, i.e., ongoing burning pain, paroxysmal pain, and mechanical dynamic allodynia.
Funding: Pfizer, Italy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropathic pain is due to a lesion or disease of the somatosensory nervous system [1]. Besides clinical examination, diagnostic tests assessing non-nociceptive and nociceptive afferent pathways are useful in patients with suspected neuropathic pain, as they provide definite evidence of somatosensory nervous system damage. Different diseases of the peripheral and central nervous system may cause neuropathic pain (e.g.. diabetic neuropathy, postherpetic neuralgia, multiple sclerosis) [1, 2].
Neuropathic pain manifests as a range of different symptoms, including ongoing burning pain, pain similar to squeezing or pressure, paroxysmal electric shock-like sensations or stabbing pain, and mechanical dynamic allodynia [1]. This article summarizes the available methods for assessing neuropathic pain and discusses the potential pathophysiology underlying various neuropathic pain phenotypes.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by the author.
Pain Assessment
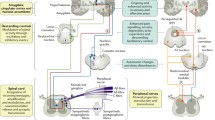
There are several available methods for the assessment of somatosensory afferent pathways in patients with suspected neuropathic pain (Fig. 1).
Quantitative Sensory Testing
Quantitative sensory testing (QST) is a psychophysical technique that measures perception in response to controlled skin stimuli of ascending and descending orders of magnitude in patients with suspected neuropathic pain [3]. QST enables clinicians to assess small-fiber neuropathies (SFNs), which are often misdiagnosed with clinical examination and not apparent on standard nerve-conduction studies. QST measures the perception of mechanical, thermal, and painful stimuli, and is particularly suited to assess mechanical and thermal allodynia and hyperalgesia. However, as sensory abnormalities are often reported in non-neuropathic pain, QST is insufficient to determine differential diagnoses [3].
Neurophysiological Techniques
Neurophysiological techniques include nerve conduction studies and measurement of somatosensory-evoked potentials (SEPs), trigeminal reflexes, laser-evoked potentials (LEPs), contact-heat-evoked potentials (CHEPs), and microneurography [3, 4]. These techniques assess large non-nociceptive and small nociceptive afferent fibers, and are therefore useful in the diagnosis of central nervous system (CNS) and peripheral nervous system (PNS) diseases.
Standard nerve conduction studies and SEPs are considered to be first-line techniques in patients with suspected neuropathic pain [4]. The standard nerve conduction study is the reference standard technique for a definite diagnosis of peripheral neuropathy. SEPs are used to assess patients with CNS disorders, such as multiple sclerosis and spinal cord injury [5].
Trigeminal reflexes are considered the best neurophysiological tool for investigating patients with trigeminal disease. They consist of different reflex responses. The most widely used trigeminal reflex responses are the blink reflex and the masseter inhibitory reflex; these responses allow for assessing the three trigeminal nerve divisions [6]. Although nerve conduction studies, somatosensory evoked potentials, and trigeminal reflexes are widely used for assessing somatosensory afferent pathway damage, they are responses mediated by non-nociceptive fibers, and thus they do not provide any information on the nociceptive system.
LEPs and CHEPs are widely used for investigating the nociceptive system. Laser-generated heat pulses and contact heat stimuli selectively activate Aδ and C mechano-thermal nociceptors, evoking scalp potentials associated with Aδ fiber activation [3,4,5].
Microneurography is a minimally invasive method for recording action potential from nociceptive C axons [3]. However, this technique is time consuming, has limited availability, and requires an expert investigator and a cooperative patient; therefore, its use in routine practice is not yet well established.
Skin Biopsy
Punch skin biopsies with immunostaining allow for visualization and assessment of intraepidermal nerve fiber (IENF) density [3]. Biopsies are analyzed by bright-field immunohistochemistry or immunofluorescence, typically with antibodies against the protein gene product 9.5, a nonspecific panaxonal marker [7, 8]. Decreased IENF density is indicative of SFN, and is a reliable method of establishing this diagnosis [7]. Biopsies are commonly used to investigate small-fiber involvement in patients with diabetic neuropathy, or neuropathy of infectious or inflammatory etiology [7, 8]. However, the relationship between skin biopsy data and neuropathic pain is complex; IENF density may be associated with the existence of neuropathic pain, but does not correlate with pain intensity [9].
Types of Neuropathic Pain
Ongoing Burning Pain
Ongoing burning pain is one of the most representative types of neuropathic pain. In patients with distal symmetrical peripheral neuropathy, the burning pain results from hyperexcitability of irritable nociceptors or regenerating nerve sprouts [1]. Microneurography has shown that, in patients with length-dependent painful neuropathy (e.g., postherpetic neuralgia or radiculopathy), the ongoing pain is caused by abnormal spontaneous C fiber activity [10, 11]. In other neuropathic pain conditions, the cause is anatomical denervation in which a primary lesion affects the neuronal cell body or postganglionic axon exposing the postsynaptic membrane of the second-order neuron to local transmitters, consequently causing spontaneous firing [1]. This process, called “denervation supersensitivity”, is associated with burning pain [12].
Paroxysmal Pain
Paroxysmal pain is often described as a shooting, electric-shock like or stabbing sensation. Previous studies have associated paroxysmal electric shock-like with non-nociceptive Aβ fiber abnormalities in patients with postherpetic neuralgia or carpal tunnel syndrome [13, 14]. In these patients, neurophysiological test findings suggest that the paroxysmal pain may originate from focal demyelination of Aβ fibers [13, 14].
In trigeminal neuralgia, the most representative neuropathic pain condition manifesting with paroxysmal pain, focal compression by aberrant vessels or benign tumors mechanically damages large myelinated fibers and causes demyelination [15, 16]. Aβ fiber demyelination increases the susceptibility of neurons to ectopic excitation and high-frequency discharges, which leads to typical paroxysmal pain [15, 16].
Allodynia
Allodynia is the experience of pain from a non-painful stimulation of the skin, such as light touch; this is in contrast to hyperalgesia, which is experiencing more intense pain than would be expected from stimuli that normally cause pain. Dynamic mechanical allodynia (i.e., elicited by light, moving, tactile stimuli) develops when a pain pathway lesion causes changes in the reactivity of central nociceptive neurons, such that they respond to low-threshold Aβ afferent fibers [1, 12]. Examples of dynamic mechanical allodynia include pain caused by skin contact with clothing in patients with postherpetic neuralgia, or contact between feet and bed sheets in patients with peripheral neuropathy. Dynamic mechanical allodynia therefore appears to be caused by Aβ fiber abnormalities [1]. Selective blockade of A fiber signaling in patients with neuropathic pain abolishes allodynia, but has no effect on burning pain, which is mediated by C fiber afferents [17]. The slow conduction velocity through Aδ and C fibers means these fibers are unlikely to contribute to the initial explosive onset of dynamic allodynia, but their hyperexcitability may modulate and maintain the ongoing after-sensations and play a role in the observed CNS changes [1]. This hypothesis is supported by studies showing that allodynia is relieved by topical lidocaine in patients with postherpetic neuralgia [18, 19].
Most investigators consider allodynia to be a CNS phenomenon occurring as a result of central sensitization, but others propose that allodynia is caused by peripheral sensitization. Indirect support for the latter hypothesis comes from studies using QST and LEPs. QST testing shows that thermal-pain sensation is preserved in many patients with postherpetic neuralgia who experience allodynia [1]. A previous study showed that patients with painful neuropathy and allodynia have partially preserved LEPs compared with patients with painful neuropathy and no allodynia [1]. However, the most convincing evidence that sensitized peripheral nociceptors are the primary determinant of allodynia comes from microneurographic studies. These studies showed that allodynia occurs secondary to abnormal firing of C nociceptors in response to light mechanical stimulation [10].
Conclusion
Diagnostic tests reliably provide evidence of somatosensory afferent pathway damage, thus supporting the diagnosis of neuropathic pain.
Neuropathic pain manifests with different symptoms. Evidence suggests that each symptom is mediated by a distinct mechanism. This has implications for treatment. Pharmacological treatment should be targeted to the specific mechanism.
References
Truini A, Garcia-Larrea L, Cruccu G. Reappraising neuropathic pain in humans—how symptoms help disclose mechanisms. Nat Rev Neurol. 2013;9(10):572–82.
Cohen J, Fadul C, Jenkyn L, Ward T. Disorders of the nervous system: Chapter 19—Pain. 2008. https://www.dartmouth.edu/~dons/part_2/chapter_19.html#chpt_19_deafferentation. Accessed 28 Apr 2017.
Cruccu G, Sommer C, Anand P, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17(8):1010–8.
Garcia-Larrea L. Objective pain diagnostics: clinical neurophysiology. Neurophysiol Clin. 2012;42(4):187–97.
Cruccu G, Aminoff MJ, Curio G, et al. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol. 2008;119(8):1705–19.
Cruccu G, Deuschl G. The clinical use of brainstem reflexes and hand-muscle reflexes. Clin Neurophysiol. 2000;111(3):371–87.
Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17(7):903–12 (e44–9).
Vlckova-Moravcova E, Bednarik J, Belobradkova J, Sommer C. Small-fibre involvement in diabetic patients with neuropathic foot pain. Diabet Med. 2008;25(6):692–9.
Truini A, Biasiotta A, Di Stefano G, et al. Does the epidermal nerve fibre density measured by skin biopsy in patients with peripheral neuropathies correlate with neuropathic pain? Pain. 2014;155(4):828–32.
Kleggetveit IP, Namer B, Schmidt R, et al. High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain. 2012;153(10):2040–7.
Ochoa JL, Campero M, Serra J, Bostock H. Hyperexcitable polymodal and insensitive nociceptors in painful human neuropathy. Muscle Nerve. 2005;32(4):459–72.
Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429(1–3):23–37.
Truini A, Galeotti F, Haanpaa M, et al. Pathophysiology of pain in postherpetic neuralgia: a clinical and neurophysiological study. Pain. 2008;140(3):405–10.
Truini A, Padua L, Biasiotta A, et al. Differential involvement of A-delta and A-beta fibres in neuropathic pain related to carpal tunnel syndrome. Pain. 2009;145(1–2):105–9.
Burchiel KJ. Abnormal impulse generation in focally demyelinated trigeminal roots. J Neurosurg. 1980;53(5):674–83.
Burchiel KJ. Ectopic impulse generation in focally demyelinated trigeminal nerve. Exp Neurol. 1980;69(2):423–9.
Koltzenburg M, Torebjork HE, Wahren LK. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain. 1994;117(Pt 3):579–91.
Rowbotham MC, Davies PS, Fields HL. Topical lidocaine gel relieves postherpetic neuralgia. Ann Neurol. 1995;37(2):246–53.
Rowbotham MC, Davies PS, Verkempinck C, Galer BS. Lidocaine patch: double-blind controlled study of a new treatment method for post-herpetic neuralgia. Pain. 1996;65(1):39–44.
Acknowledgements
This supplement has been sponsored by Pfizer, Italy. The article processing charges for this publication were also funded by Pfizer, Italy. Andrea Truini thanks Cécile Duchesnes, PhD, of Springer Healthcare Communications who wrote the outline, and Sarah Greig, PhD, of Springer Healthcare Communications who wrote the first draft of this manuscript. This medical writing assistance was funded by Pfizer, Italy. The named author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, takes responsibility for the integrity of the work as a whole, and has given final approval for the version to be published.
Disclosures
Andrea Truini has received honorarium from Pfizer, as well as research grant, consulting fees, and/or payments for lectures from Alfasigma Group, Angelini, and Gruenenthal.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by the author.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/20DCF060253B492B.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Truini, A. A Review of Neuropathic Pain: From Diagnostic Tests to Mechanisms. Pain Ther 6 (Suppl 1), 5–9 (2017). https://doi.org/10.1007/s40122-017-0085-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-017-0085-2