Abstract
Introduction
The burden of respiratory syncytial virus (RSV), which causes acute respiratory illness, is well recognized among the pediatric population but also imposes a significant risk to the elderly (age ≥ 60) and those with underlying comorbidities. The study aimed to review the most recent data on epidemiology and burden (clinical and economic) of RSV in the elderly/high-risk populations in China, Japan, South Korea, Taiwan, and Australia.
Methods
A targeted review was conducted of English, Japanese, Korean, and Chinese language articles published from 1 January 2010 to 7 October 2020 relevant for the purpose.
Results
A total of 881 studies were identified, and 41 were included. The median proportion of elderly patients with RSV in all adult patients with acute respiratory infection (ARI) or community acquired pneumonia was 79.78% (71.43–88.12%) in Japan, 48.00% (3.64–80.00%) in China, 41.67% (33.33–50.00%) in Taiwan, 38.61% in Australia, and 28.57% (22.76–33.33%) in South Korea. RSV was associated with a high clinical burden on those patients with comorbidities such as asthma and chronic obstructive pulmonary disease. In China, inpatients with ARI showed a significantly higher rate of RSV-related hospitalization than outpatients (13.22% versus 4.08%, p < 0.01). The median length of hospital stay among elderly patients with RSV was longest in Japan (30 days) and shortest in China (7 days). Mortality data varied by region with some studies reporting rates as high as 12.00% (9/75) in hospitalized elderly patients. Finally, data on the economic burden was only available for South Korea, with the median cost of a medical admission for an elderly patient with RSV being US dollar (USD) 2933.
Conclusion
RSV infection is a major source of disease burden among elderly patients, especially in regions with aging populations. It also complicates the management of those with underlying diseases. Appropriate prevention strategies are required to reduce the burden among the adult, especially the elderly, population. Data gaps regarding economic burden of RSV infection in the Asia Pacific region indicates the need for further research to increase our understanding on the burden of this disease in this region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Respiratory syncytial virus infection (RSV) is a major source of disease burden among elderly patients, especially in regions with aging populations. |
Proportion of elderly patients with RSV among those with acute respiratory infection or community acquired pneumonia in countries from the Asia Pacific region ranged from 28.57% in South Korea to 88.12% in Japan. |
RSV is associated with a high clinical burden in patients with comorbidities such as asthma and chronic obstructive pulmonary disease. |
RSV infection places a heavy burden on hospital resource use such as hospital or intensive care unit stay, or supplemental oxygen therapy and ventilation, especially among older adults. |
Introduction
Respiratory syncytial virus (RSV) causes acute respiratory illness both in young children and adults, including individuals who are immunocompromised [1, 2] or have underlying chronic respiratory and heart diseases [3,4,5,6], and who are elderly [7, 8]. Prevalence of RSV is heterogeneous [9], as it depends on several factors, including sampling methods, quality of the specimen, frequency of swab testing, and the techniques used for the test [10,11,12,13].
Evidence from international studies has emerged in the recent years showing that RSV is a frequent cause of viral disease in hospitalized adults aged 65 years or more, with a disease burden of 41 disability-adjusted life years (DALYs) per 1000 population [14]. Although RSV is the cause for many hospitalizations of elderly patients with moderate-to-severe influenza-like illness (ILI), the global burden of disease in this population is not well known [15]. Limited evidence suggests that RSV might be particularly burdensome among the elderly living in community-dwelling arrangements (i.e., nursing home residents) [3, 16]. Elderly are also at an increased risk of morbidity and mortality, as the presence of comorbidities would lower their immune system and expose them not only to RSV but also other coinfections [17], which lead to more complex clinical management, worse health outcomes, and higher healthcare costs [18].
The burden of RSV infection among the elderly (age ≥ 60) and the high-risk populations (e.g., those with cardiac or respiratory comorbidities) in Asia Pacific is under documented. A systematic review and meta-analysis by Pangesti et al. (2019) focused on the epidemiology of RSV in Western Pacific [19]. However, the review mainly focused on the prevalence, seasonality, and genotypes of RSV among infants, children, and younger adults.
The present literature review aimed to identify the most recent data on epidemiology, clinical characteristics, and economic burden of RSV in the elderly/high-risk population in Taiwan, China, Japan, South Korea, and Australia.
Methods
A structured search was conducted for English-language articles published from 1 January 2010 to 7 October 2020. Where data are scarce for an individual region, the timeframe was expanded to previous years with available evidence (e.g., a study published in 2005 was included for Taiwan, since only one other study was included). Search terms were developed according to the PICOS framework (Population, Intervention, Comparator, Outcomes, and Study type) outlined in the supplemental file. Searches were conducted on Embase, Medline, the Chinese language databases Wanfang and CNKI, the Korean databases Koreamed and kmbase, and the Japanese database Ichusi-web. Furthermore, supplementary hand searching in Google Scholar was conducted, and review articles were cross-referenced to identify additional articles. Finally, databases and reports linked to sentinel surveillance systems for RSV in each region were searched to obtain relevant epidemiology data.
Search strings included, but were not limited to, “respiratory syncytial virus,” “syncytial virus,” “RSV incidence ,” “mortality,” “prevalence,” “hospitalization,” “survival,” “incidence rate,” “death,” “mechanical ventilation,” “epidemiology,” and “transmission.” An example of the full version of search terms for Embase is presented in the supplemental file. Study selection was done based on predefined selection criteria following the PICOS framework. Titles and abstracts were screened, and quality checked independently. Those included at the title and abstract screening stage were full text reviewed and further assessed for eligibility.
The geographical focus of this review was on Taiwan, China, South Korea, Japan, and Australia, covering approximately 87% of the total population in the Western Pacific region [20, 21]. Studies conducted in elderly patients (≥ 60 years), or adult patients with defined underlying conditions that reported the outcomes of interest, were included. Observational studies and surveillance reports were included. Case reports, narrative reviews, commentaries, modelling, and review articles were excluded.
Included studies were narratively summarized and results descriptively analyzed. No formal quantitative statistical assessment was done as part of this research.
This article is based on previously conducted studies, and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Study Selection
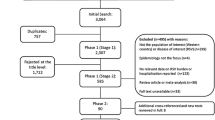
The results of all searches are summarized in the simplified PRISMA diagram shown in Fig. 1. The initial database search identified a total of 863 records, and an additional 18 articles were identified via supplementary hand searching. Following screening activities, a total of 41 studies were selected for data extraction. The main characteristics of these studies are summarized in Table 1.
Epidemiology
Figure 2 illustrates the proportions of adult patients with RSV, among all adult patients aged over 18 with acute respiratory infection (ARI) (including ILIs) and community acquired pneumonia (CAP) in each region (China 0.81–29.63%, Australia 9.07%, Japan 7.48–19.44%, South Korea 1.21–7.52%, and Taiwan 1.19–4.76%). Data are based on 28 studies conducted primarily in hospitalized patients (17 studies included inpatients only [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38], 5 studies included both inpatients and outpatients [39,40,41,42,43], 3 studies included only outpatients [44,45,46], and 3 studies did not specify hospitalization status of patients included [47,48,49]). Another 13 studies specifically focused on patients with RSV who had chronic obstructive pulmonary disease (COPD) or asthma [50,51,52,53,54,55,56,57,58,59,60,61,62], and for which it was inappropriate to compare with the previous 28 studies.
Percentage of RSV infection among all adults hospitalized with an acute respiratory infection (ARI) (including ILIs) and community acquired pneumonia (CAP) in China, Japan, South Korea, Taiwan, and Australia. *Data for Japan, Australia, South Korea, and China are available from the World Bank Open Data. Year 2020: https://data.worldbank.org/indicator/SP.POP.65UP.TO.ZS?name_desc=false. Year 2030: https://databank.worldbank.org/source/health-nutrition-and-population-statistics:-population-estimates-and-projections. Data for Taiwan are available from the interior statistics of Taiwan governmental websites. Year 2020: https://ws.moi.gov.tw/001/Upload/OldFile/site_stuff/321/1/month/month_en.html. Year 2030: https://pop-proj.ndc.gov.tw/main_en/dataSearch.aspx?uid=78&pid=78
Results varied mainly due to different inclusion criteria, sample sizes, and patient subgroups. Most notably, RSV detection rate varied largely in the Chinese studies. For example, Ju et al. (2014) [28] [0.81% (1/124)] was conducted in a southern city, while Wang et al. (2018) [23] [29.63% (56/189)] was done in a northern city with a longer winter season (when RSV is usually transmitted). This variation was also present in Japanese studies due to different sample sizes; the incidence was higher in studies with small sample sizes [19.44% (7/36)], and some studies reported specific data for inpatients [7.48% (101/1351)] and outpatients [19.10% (17/89)] [30, 40, 58, 59]. Data in South Korean studies also varied from 1.27% (9/770) in Park et al. (2017) [45] to 43.48% (30/69) in Kim et al. (2018) [32], which is likely due to different patient inclusion criteria.
Epidemiology of respiratory syncytial virus across regions in elderly population and susceptible younger adults
A total of 18 studies reported the proportion of elderly (age > 60) population infected with RSV among all adult patients with ARI or CAP (studies focusing on patients with RSV only were excluded ) (Table 1). Data were heterogeneous, with Japanese studies reporting from 71.43 to 88.12% [30, 37], followed by Chinese studies with a median proportion of 48.00% (range 3.64–80%) [22,23,24,25,26, 35, 39, 41, 42, 47], a median of 41.67% (range: 33.33–50%) in studies from Taiwan [34, 46], 38.61% in one study from Australia [49], and a median proportion of 28.57% (range 22.76–33.33%) in South Korean studies [31, 45, 48].
On the other side, the proportion of RSV infection among younger adults at high risk (i.e., with underlying asthma or COPD) among all adult patients, mostly inpatients, with lower respiratory tract infection, was between 1.52 and 4.44%.[51, 56, 59, 60, 62]. Specifically, three of these five studies evaluated adult patients (mean/median age 48.7–56.3) with asthma in China (outpatients), Japan (inpatients and outpatients), and South Korea (hospitalization status unspecified). RSV was found in 4.44% (6/135), 2.83% (3/106), and 3.41% (11/323) of the study populations, respectively [51, 56, 60]. The fourth study reviewed 278 episodes from 213 hospitalized South Korean patients (mean age 69.2, standard deviation 11.0) with COPD exacerbations, and RSV was detected in 4.32% (12/278) of them [59]. The fifth study investigated viral infection in 264 hospitalized patients with acute exacerbation of COPD in Shanghai, China, and RSV A was detected in 4 (1.52%) of them [62].
With regard to specific RSV serotypes, the Chinese study Xiang et al. (2013) reported similar infection rates and clinical manifestations between RSV A and RSV B [55.79% (53/95) versus 44.21% (42/95)] among RSV-positive patients (95 RSV-positive out of 9871 patients with ARI, median age 30, range 15–97) between 2005 and 2010 [50]. Of note, serotype prevalence varied depending on the specific timeframe analyzed [50], a phenomenon also seen in South Korea by Yoon et al. (2020), where RSV A accounted for nearly 90% of all RSV infections from 2012 to 2014, while RSV B dominated from 2014 to 2015 [58].
Epidemiology of Respiratory Syncytial Virus among the Elderly in China
In China, elderly patients with RSV represent a median proportion of 48.00% (range 3.64–80.00%) among all adult patients with ARI or CAP who had positive RSV detection [22,23,24,25,26, 35, 39, 41, 42, 47]. This wide range was mainly due to the different regional focus across studies. Nationwide, from 28,369 patients of all ages with acute lower respiratory infections (ALRI) between 2009 and 2013, a total of 7732 were tested and 1300 had confirmed viral etiology, 125 of them had detectable RSV, of which 44.00% (55/125) were over 65 years old.[22] In this same period, RSV-positive elderly patients among hospitalized patients with ALRI remained at a low level (around 2%) compared with those aged 0–64 (up to 26%). Another multicenter prospective registry study recruited 2336 adult inpatients with CAP during 2015–2017. RSV was detected in 57 patients, of which 43.86% (25/57) were over 65 years old [35].
Data also varied at subregional or provincial level, possibly derived from the differences in geographical areas, inclusion criteria, and patient groups. For example, a study conducted in a southern city of China by Ju et al. (2014) included 344 adult outpatients with ILI, with 36.05% (124/344) testing positive for a viral infection, of which only one patient (0.81%, 1/124) over 60 years old was positive for RSV [28]. In contrast, infection rate was much higher in a northern city, where 189 out of 1300 adult inpatients with lower respiratory tract infection had positive viral detection. RSV infection rate was 29.63% (56/189) among them, with 76.79% (43/56) being older adults (age ≥ 50) [23]. Additionally, RSV infection rates also differed within the same geographical area. Huang et al. (2020) and Liao et al. (2015) analyzed 22,680 and 12,502 patients with ARI, of all ages, in Southern China, respectively [26, 39]. Among the adults with positive viral detection, incidence of RSV infection among adults with ARI ranged from 1.73% (142/8,198) in Huang et al. (2020) to 25.53% (437/1,711) in Liao et al. (2015). In contrast, among RSV-positive patients, the population proportion of elderly patients was higher in Huang et al. (2020) [52.82% (75/142) and 6.86% (30/437), respectively]. Nevertheless, it should be noted that both the timeframes of each study (2009–2018 versus 2009–2014) and cut-off ages for elderly patients (60 years old versus 51 years old) were different [26, 39].
When comparing RSV infection rate between inpatients and outpatients with ARI, Yu et al. (2018) identified 37.18% (532/1431) of adult inpatients and 43.12% (2107/4886) of adult outpatients with positive viral detection. RSV accounted for 10.57% (37/350) and 5.88% (108/1836), respectively. Although not statistically significant (p > 0.26), this difference was further increased in the elderly (age ≥ 65) (59.46% for inpatients versus 9.26% for outpatients) among RSV-positive adults [41].
Finally, prevalence of RSV A was higher than RSV B in two studies [25, 50]. Observational data from a single hospital reported by Xiang et al. (2013) showed that 95 [0.96% (95/9871)] adult patients with ARI were positive for RSV, of which 25 were aged over 60, and RSV A was more frequently found than RSV B (15 versus 10) among them [50]. A similar trend was observed in another study by Liu et al. (2015), where RSV was detected in 41 out of 607 ARI patients of all ages, including 29 with RSV A and 12 with RSV B. Only four patients were aged over 60, and all of them had RSV A, while RSV B was not found in elderly patients [25].
Epidemiology of Respiratory Syncytial Virus among the Elderly in Japan
In Japan, the RSV infection rate in adult patients with ARI or CAP ranged from 7.48% to 19.44% [30, 37, 38, 44] Specifically in the elderly (age > 65), the proportions of RSV-positive patients ranged from 71.43% (5/7) to 88.12% (89/101) among all adult patients hospitalized with RSV-related CAP [30, 37]. On the other hand, another study by Saraya et al. (2017) evaluated 106 adult patients [46.23% (49/106) hospitalized] with acute asthma, and RSV was detected in 3 (2.83%) of them [56].
Epidemiology of Respiratory Syncytial Virus among the Elderly in South Korea
The RSV infection rate among adult patients with ARI (including ILI) or pneumonia ranged from 1.21% to 7.52% in South Korea, with elderly patients (≥ 60 years) taking up 22.76–33.33% (median 28.57%) of all adult RSV cases [31, 32, 36, 43, 45, 48]. A nationwide surveillance of human acute respiratory virus infections covering 36 sentinel hospitals during 2013–2015 by Kim et al. (2018) detected RSV in 2.78% (145/5217) of adult patients with respiratory viruses, and 22.76% (33/145) of them were over 65 years old [48]. Similarly, a study part of the Korea Influenza and Respiratory Viruses Surveillance System (KINRESS) found RSV positivity in 1.21% (3/248) of outpatient adults with positive virus detection, with one patient over 65 years of age [45]. A similar result was found in another study analyzing the same type of population, where RSV detection rate was 1.27% (7/553), of which 28.57% (2/7) were over 60 years old [31].
RSV A was generally more prevalent than RSV B in South Korea, with a wide range of differences (RSV A versus RSV B) across studies (68.63% versus 23.04% [58], 30.43% versus 13.04% [32], and 2.52% versus 0.39% [43]). In addition, both serotypes presented a biannual exchanging dominance over each other [58].
Epidemiology of Respiratory Syncytial Virus among the Elderly in Taiwan
Studies from Taiwan generally had small sample sizes. Shih et al. (2015) analyzed 267 adults with ARI who visited a medical center in Taiwan during 2012–2013, and RSV was detected in 6 of 126 (4.76%) patients with positive virus detection, with 2 of them (33.33%) being elderly (age ≥ 60) [46]. Lauderdale et al. (2005) analyzed the etiologic profile of 168 adult patients who were diagnosed with lower respiratory infection in 13 hospitals throughout Taiwan during 2001–2002. RSV was detected in 1 out of 2 hospitalized adults with CAP [34].
Epidemiology of Respiratory Syncytial Virus among the Elderly in Australia
In Australia, the study by Price et al. (2019) detected 9.07% (632/6970) of patients with RSV among all adults with viral infection during 2002–2017, with 38.61% (244/632) of them aged 65 years or more [49]. The same study found that RSV revealed a moderate to strong correlation between epidemic curves of influenza and RSV in 9 of the 13 years analyzed, with higher burden in the winter; nonetheless, RSV infection was negatively correlated with influenza as a coinfection [49].
Nationwide, the prevalence of RSV in elderly (age ≥ 65) patients was 6 per 100,000 Australians according to Saravanos et al. (2019) [61]. This study was based on hospitalization data with international classification of diseases codes. RSV-coded adults were found in 4.30% (2746/63,814) of patients of all ages between 2006 and 2015, among which 63.44% (1742/2746) were over 65 years old. The study also found a rising trend of RSV-coded hospitalization rate in adults, especially among those ≥ 65 years old (from around 1/100,000 population in 2006 to around 19/100,000 population in 2015) [61].
Clinical Burden
The prevalence of comorbidities, mean length of hospital stay (days), frequency of intensive care unit (ICU) admission of elderly patients with RSV among all patients, and the 30-day hospital mortality are summarized in Tables 2 and 3. Our review found that South Korea and China reported most clinical burden data on hospitalizations, in-hospital mortality, length of stay, and need for ICU. However, there is an information gap regarding length of stay and ICU frequency in Taiwan, and ICU frequency is not identified in Japan.
Comorbidities and Complications
Cardiac disease, COPD, and asthma, as well as complications such as pneumonia, were commonly reported along with RSV in infected elderly patients (Table 2). The evidence identified during this review suggests that the presence of these comorbidities increases the risk of complications leading to hospitalization. The prevalence of several comorbidities on ARI or patients with RSV was available for all regions, except Australia.
Cardiac disease was present in 37.25% (19/51) and 27.27% (36/132) of adult patients with RSV infection from China and South Korea, respectively [53, 58]. Additionally, the Chinese study Zhang et al. (2020) found that the presence of cardiovascular diseases was significantly higher in patients with RSV than patients with influenza [50.98% (26/51) versus 34.41% (96/279), p = 0.024] [53].
Similar to cardiac diseases, Zhang et al. (2020) found exacerbation of asthma to be more frequent in the RSV group than in the influenza group (p = 0.021) [53]. In addition, respiratory virus infection (e.g., RSV, rhinovirus, influenza) caused exacerbation of asthma in the Chinese study by Liao et al. (2016) but the contribution of RSV was not reported separately (Table 4), with respiratory virus infection rate in the asthma exacerbation group being significantly higher than in the stable asthma group [34.29% (24/70) versus 18.46% (12/65), p = 0.038], and the healthy control group [34.29% (24/70) versus 13.43% (18/134), p < 0.001] [51]. In contrast, the South Korean study by Seo et al. (2017) did not confirm this finding (Table 4), as nonsignificant differences were found between both groups [26.25% (68/259) versus 18.75% (12/64), p = 0.213]. In this case, the contribution of RSV was also reported and no significant differences were found [3.09% (8/259) versus 4.69% (3/64), p = 0.220] [60]. Finally, similar findings were shown by Kwak et al. (2016) with regards to COPD exacerbation in South Korean patients, where no significant difference was found between patients with positive or negative respiratory virus (p-value not reported). Interestingly, females were found to be at higher risk of respiratory viral infections in COPD exacerbations than males [odds ratio 2.58, 95% confidence interval (CI) 1.25–5.31] in multivariate regression analysis adjusting for sex, age, body mass index, lung function, and history of exacerbations (p = 0.010) [59].
Lastly, pneumonia was found to be a complication in more than 40.00% of hospitalized adults with RSV infection [52, 53, 55, 58]. Once more, Zhang et al. (2020) found that the proportion of pneumonia was significantly higher in the RSV group than in the influenza group [58.82% (30/51) versus 38.35% (107/279), p = 0.006] [53].
Hospitalizations and Intensive Care Unit Admissions
The length of hospitalization of patients with RSV infection across all regions, excluding Taiwan, are summarized in Table 3. Overall, these studies indicated that RSV infection places a heavy burden on hospital resource use (e.g., hospital/ICU stay, supplemental oxygen therapy, and ventilation), especially among older adults.
In China, the study by Huang et al. (2020) found that patients with ARI (of all ages) requiring hospital stay had significantly higher RSV infection rate than those who did not need hospitalization [13.22% (2049/15,504) versus 4.08% (293/7,176), p < 0.01] [39]. However, specifically for the elderly (aged > 60), Ye et al. (2017) did not find different RSV infection rates between outpatients [2.47% (9/365)] and inpatients [1.64% (10/611)] with ARI (p = 0.269) [40]. In Australia, the national population-level analysis by Saravanos et al. (2019) reported that 60.89% (6558/10,771) of adults in hospital with a RSV code were patients aged 65 years or older [61].
The longest hospital stay among elderly patients with RSV infection was seen in Japan, with a mean duration of 30 (range 4–115) days, reported by Takahashi et al. (2016) (n = 54) [55]. On the other hand, the Chinese study Lee et al. (2013), which included 607 older adults with a mean age of 75.1 [standard deviation (SD) 16.4], found that the median hospital stay was 7 days [interquartile range (IQR) 5–14] [52].
In hospitalized older adults with respiratory viral infection, the proportion of cases requiring supplemental oxygen therapy among all patients with RSV infection was 79.63% (43/54) in a Japanese study [55]. A significantly higher proportion was reported among patients with RSV infection than those with influenza infection, according to Lee et al. (2013) from South Korea [67.87% (412/607) versus 59.04% (323/547); p = 0.002] [52].
The need for intensive care among hospitalized RSV-infected elderly patients was considerable, ranging from 6.67% (1/15) to 37.93% (11/29) (median across studies: 25.10%), and mechanical ventilation was also frequently required [24, 33, 53, 58]. The highest ICU admission rate for patients with RSV was found in China [37.93% (11 out of 29 RSV-positive severe ARI patients)] [24] and the lowest was in Australia [6.67% (1 out of 15 older RSV-patients)] [33], with South Korea showing middle rates [26.67% (20 out of 75 older RSV-patients)] [58]. Mechanical ventilation was needed in 14.77% (11/75) of patients in the South Korean study [58].
In-Hospital Mortality Rate
Studies conducted in China, Japan, South Korea, and Australia indicated that RSV infection in elderly patients was associated with a median (across studies) in-hospital all-cause mortality rate of 7.35% (range 4.71–12.00%) (Table 3). Specifically, the 30-day all-cause mortality rate in China was 9.06% (55/607), with 60-day mortality among the elderly being 11.86% (72/607) [52]. Consistent data were found for Japan, South Korea, and Australia, with in-hospital all-cause mortality rate of elderly patients with RSV available for each region [5.56% (3/54), 12.00% (9/75), and 4.71% (82/1742), respectively] [55, 58, 61].
Additionally, a study from South Korea with a small sample size suggested that RSV infection was not related to a significantly higher in-hospital mortality, since the 28-day ICU mortality did not differ significantly between patients with and without RSV [20.00% (2/10) versus 35.64% (67/188), p = 0.50] [36].
Economic Burden
As outlined above, RSV is associated with considerable clinical burden, particularly for patients with preexisting chronic conditions, and accounts for a considerable proportion of the burden faced by primary and secondary care systems in terms of hospital stays and use of ICU resources. However, economic burden of RSV infection was only reported in the South Korean study by Yoon et al. (2020), where the median direct medical cost of a hospital admission, such as bed fee, medicines, and any medical procedures, for an elderly patient with RSV was US dollar (USD) 2933 (IQR 1748–6340). Although statistically insignificant, the median cost of medical admission showed an increasing trend along with age (USD 1,957 for age group of 19–49, USD 2116 for age group of 50–64, and USD 2,933 for age group ≥ 65) [58].
Discussion
Our review identified a large variation in results within the regions of interest, potentially driven by differences in study design and geographical area, as most studies were local, regional or provincial. Nationwide RSV surveillance data for high-risk/elderly patients was only available for China [22], South Korea [48], and Australia [61]. Considering these factors, RSV infection rate is likely to be underestimated. Furthermore, seasonal variability across the regions and timeframe of the studies could explain some of the differences found, as shown in a recent report by the Global Influenza Surveillance and Response System (GISRS) led by the World Health Organization (WHO) [63].
Aging is an important factor to influence the clinical burden of RSV infection. As shown in Fig. 2, the percentage of people aged above 65 is increasing in all five regions, thus disease burden of RSV is likely to be increased with the aging of their population. Besides, our findings are in line with recently published studies investigating the burden of RSV in older adults. Lin et al. (2021) analyzed the etiologic agents of 212 adult patients (in which 119 were aged ≥ 65) hospitalized with CAP in two major hospitals in northern Taiwan during 2016–2018, and RSV was identified in four patients (5.19%, 4/77), with two (50.00%, 2/4) of them aged over 65 years [64]. More broadly, He et al. (2021) reported that respiratory virus infection could result in higher overall mortality rate [(8.20% (5/61) versus 1.23% (2/162), p = 0.026] in older patients (> 65 years old) with CAP than younger patients with CAP (≤ 65 years old) [65].
Our review also highlighted how RSV burden may be compounded by the presence of chronic diseases, which are common among the elderly. Two literature reviews by Kurai et al. (2013) and Britto et al. (2017) suggested that RSV is one of the major causes of exacerbation in patients with asthma or COPD, and their clinical outcome such as respiratory function and life expectancy are generally poor [66, 67]. Consistently, findings from this review suggest that RSV infection was accountable for additional clinical burden (i.e., complications, hospital stay, in-hospital mortality) of adult, especially the elderly, patients with underlying diseases such as COPD and asthma.
Compared with Asia Pacific regions, surveillance data regarding the epidemiology of RSV infection are better documented in many European countries. For example, RSV incidence rate in the population aged ≥ 60 was approximately 2.67/100,000 population in Germany during 2017–2019 according to the Robert Koch Institute [68], and 7.73/100,000 in England and Wales based on surveillance data from Public Health England during 2019–2020 [69]. The French Public Health Agency and the European Centre for Disease Prevention and Control reported 12,929 confirmed cases of RSV during 2016–2019 from non-sentinel sources [70]. All countries showed an increasing trend of RSV incidence in adults, especially the elderly. Although epidemiology data is not directly comparable between Asian and European countries, it is likely that data from this review are underestimates of the true situation and the attributable burden. This suggests that there is a large and unmet need for innovative treatments that can reduce the risk of RSV in these vulnerable populations.
Several data gaps were identified while carrying out this review. While there were no strong/highly relevant data available on costs for all the regions included, there was also limited data available for the rest of measures as shown in Table 5.
Additional limitations should be kept in mind when interpreting the overall results. Data were limited despite the number of studies identified, and heterogeneity in study design limited comparability across studies and geographical regions. These aspects constrained the feasibility of conducting a meta-analysis, therefore, we provided here a descriptive analysis of the results. Quantitative analyses on the topic are warranted in future research should data gaps be minimized, for which large, nationwide, population-based studies that allow comparison in the five countries of interest are needed. Further data on the economic burden of RSV in elderly and high-risk populations in this region are also needed.
Conclusions
Our work reviews the epidemiological, clinical, and economic data related to RSV-associated infections in elderly and high-risk populations in China, Japan, South Korea, Taiwan, and Australia. The comprehensive search terms and databases covered in the study identification process supports quantity and quality of included studies. Findings from this review could potentially serve as references for healthcare decision-makers in these regions. At the same time, the data gaps identified could be a priority for researchers and public health officials to address in the future.
References
Anderson NW, Binnicker MJ, Harris DM, Chirila RM, Brumble L, Mandrekar J. Morbidity and mortality among patients with respiratory syncytial virus infection: a 2-year retrospective review. Diagn Microbiol Infect Dis. 2016;85(3):367–71.
Billings JL, Hertz MI, Wendt CH. Community respiratory virus infections following lung transplantation. Transpl Infect Dis. 2001;3(3):138–48.
Falsey AR, McElhaney JE, Beran J, van Essen GA, Duval X, Esen M, et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis. 2014;209(12):1873–81.
Dowell SF, Anderson LJ, Gary HE Jr, Erdman DD, Plouffe JF, File TM Jr, et al. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174(3):456–62.
Mehta J, Walsh EE, Mahadevia PJ, Falsey AR. Risk factors for respiratory syncytial virus illness among patients with chronic obstructive pulmonary disease. COPD J Chronic Obstr Pulm Dis. 2013;10(3):293–9.
Chaw PS, Wong SWL, Cunningham S, Campbell H, Mikolajczyk R, Nair H, et al. Acute lower respiratory infections associated with respiratory syncytial virus in children with underlying congenital heart disease: systematic review and meta-analysis. J Infect Dis. 2020;222(Supplement_7):S613–9.
McClure D, Kieke B, Armstrong L, Houghton R, Aitken S, Nyimbili E. Seasonal incidence of medically attended respiratory syncytial virus infection in a community cohort of adults ≥50 years old. PLoS ONE. 2014;9(7): e102586.
Kothe H, Bauer T, Marre R, Suttorp N, Welte T, Dalhoff K. Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J. 2008;32(1):139–46.
Tin Tin Htar M, Yerramalla MS, Moïsi JC, Swerdlow DL. The burden of respiratory syncytial virus in adults: a systematic review and meta-analysis. Epidemiol Infect. 2020;148:e48.
Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med [Internet]. 2020. https://doi.org/10.1056/NEJMc2016359.
Hou N, Wang K, Zhang H, Bai M, Chen H, Song W, et al. Comparison of detection rate of 16 sampling methods for respiratory viruses: a Bayesian network meta-analysis of clinical data and systematic review. BMJ Glob Health. 2020;5(11): e003053.
Zhang N, Wang L, Deng X, Liang R, Su M, He C, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92(4):408–17.
Suntarattiwong P, Mott JA, Mohanty S, Sinthuwattanawibool C, Srisantiroj N, Patamasingh Na Ayudhaya O, et al. Feasibility and performance of self-collected nasal swabs for detection of influenza virus, respiratory syncytial virus, and human metapneumovirus. J Infect Dis. 2021;224(5):831–8.
Gaunt ER, Harvala H, McIntyre C, Templeton KE, Simmonds P. Disease burden of the most commonly detected respiratory viruses in hospitalized patients calculated using the disability adjusted life year (DALY) model. J Clin Virol. 2011;52(3):215–21.
WHO. WHO Technical Meeting on Piloting RSV Surveillance based on the Global Influenza Surveillance and Response System. Geneva, Switzerland. 2016.
Sundaram ME, Meece JK, Sifakis F, Gasser Jr. RA, Belongia EA. Medically attended respiratory syncytial virus infections in adults aged ≥50 years: clinical characteristics and outcomes. CID. 2014;58:342–9.
Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology. 2008;23:64–74.
Valderas J, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7(4):357–63.
Pangesti KNA, El Ghany MA, Kesson AM, Hill-Cawthorne GA. Respiratory syncytial virus in the Western Pacific Region: a systematic review and meta-analysis. J Glob Health. 2019;9(2): 020431.
World Health Organization. About WHO in the Western Pacific [Internet]. [cited 2022 Jan 10]. https://www.who.int/westernpacific/about.
United Nations. World Population Prospects 2019 [Internet]. [cited 2002 Jan 10]. https://population.un.org/wpp/Graphs/DemographicProfiles/Line/935.
Feng L, Li Z, Zhao S, Nair H, Lai S, Xu W, et al. Viral etiologies of hospitalized acute lower respiratory infection patients in China, 2009–2013. PLoS ONE [Internet]. 2014;9(6). https://www.embase.com/search/results?subaction=viewrecord&id=L373399393&from=export.
Wang Y, Dong T, Qi G, Qu L, Liang W, Qi B, et al. Prevalence of common respiratory viral infections and identification of adenovirus in hospitalized adults in Harbin, China 2014 to 2017. Front Microbiol [Internet]. 2018;9(NOV). https://www.embase.com/search/results?subaction=viewrecord&id=L625247103&from=export.
Xu W, Guo L, Dong X, Li X, Zhou P, Ni Q, et al. Detection of viruses and mycoplasma pneumoniae in hospitalized patients with severe acute respiratory infection in Northern China, 2015–2016. Jpn J Infect Dis. 2018;71:134–9.
Liu T, Li Z, Zhang S, Song S, Julong W, Lin Y, et al. Viral Etiology of acute respiratory tract infections in hospitalized children and adults in Shandong Province, China. Virol J [Internet]. 2015;12(1). https://www.embase.com/search/results?subaction=viewrecord&id=L606397605&from=export.
Liao X, Hu Z, Liu W, Lu Y, Chen D, Chen M, et al. New epidemiological and clinical signatures of 18 pathogens from respiratory tract infections based on a 5-year study. PLoS ONE [Internet]. 2015;10(9). https://www.embase.com/search/results?subaction=viewrecord&id=L606939514&from=export.
Chan PKS, Tam WWS, Lee TC, Hon KL, Lee N, Chan MCW, et al. Hospitalization incidence, mortality, and seasonality of common respiratory viruses over a period of 15 years in a developed subtropical city. Med U S. 2015;94(46): e2024.
Ju X, Fang Q, Zhang J, Xu A, Liang L, Ke C. Viral etiology of influenza-like illnesses in Huizhou, China, from 2011 to 2013. Arch Virol. 2014;159(8):2003–10.
Ma HM, Lee KP, Woo J. Predictors of viral pneumonia: The need for viral testing in all patients hospitalized for nursing home-acquired pneumonia. Geriatr Gerontol Int. 2013;13(4):949–57.
Katsurada N, Suzuki M, Aoshima M, Yaegashi M, Ishifuji T, Asoh N, et al. The impact of virus infections on pneumonia mortality is complex in adults: a prospective multicentre observational study. BMC Infect Dis. 2017;17(1):755.
Park K, Kim D, Seong J, Shin I, Hong J, Park S, et al. Epidemiological features and genetic variation of human respiratory syncytial virus (HRSV) infection in Chungnam. Korea Biomed Res India. 2017;28(2):967–72.
Kim HJ, Choi SM, Lee J, Park YS, Lee CH, Yim JJ, et al. Respiratory virus of severe pneumonia in South Korea: Prevalence and clinical implications. PLoS ONE [Internet]. 2018;13(6). https://www.embase.com/search/results?subaction=viewrecord&id=L622630523&from=export.
Teh BW, Worth LJ, Harrison SJ, Thursky KA, Slavin MA. Risks and burden of viral respiratory tract infections in patients with multiple myeloma in the era of immunomodulatory drugs and bortezomib: experience at an Australian Cancer Hospital. Support Care Cancer. 2015;23(7):1901–6.
Lauderdale TL, Chang FY, Ben RJ, Yin HC, Ni YH, Tsai JW, et al. Etiology of community acquired pneumonia among adult patients requiring hospitalization in Taiwan. Respir Med. 2005;99(9):1079–86.
Zhou F, Wang Y, Liu Y, Liu X, Gu L, Zhang X, et al. Disease severity and clinical outcomes of community-acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicentre prospective registry study from the CAP-China Network. Eur Respir J [Internet]. 2019;54(2). https://www.embase.com/search/results?subaction=viewrecord&id=L2002707766&from=export.
Choi SH, Hong SB, Ko GB, Lee Y, Park HJ, Park SY, et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med. 2012;186(4):325–32.
Takaki M, Nakama T, Ishida M, Morimoto H, Nagasaki Y, Shiramizu R, et al. High incidence of community-acquired pneumonia among rapidly aging population in Japan: a prospective hospital-based surveillance. Jpn J Infect Dis. 2014;67(4):269–75.
石黒卓, 高柳昇, 高橋孝, 米田紘一郎, 宮原庸介, 徳永大道, et al. 成人市中肺炎におけるウイルス感染の関与 単一施設での前向き検討. Vol. 49, 日本呼吸器学会雑誌. 2011. p. 10–9.
Huang XB, Yuan L, Ye CX, Zhu X, Lin CJ, Zhang DM, et al. Epidemiological characteristics of respiratory viruses in patients with acute respiratory infections during 2009–2018 in southern China. Int J Infect Dis. 2020;98((Huang X.-B.) Department of Pulmonary and Critical Care Medicine, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China):21–32.
Ye C, Zhu W, Yu J, Li Z, Fu Y, Lan Y, et al. Viral pathogens among elderly people with acute respiratory infections in Shanghai, China: Preliminary results from a laboratory-based surveillance, 2012–2015. J Med Virol. 2017;89(10):1700–6.
Yu J, Xie Z, Zhang T, Lu Y, Fan H, Yang D, et al. Comparison of the prevalence of respiratory viruses in patients with acute respiratory infections at different hospital settings in North China, 2012–2015. BMC Infect Dis [Internet]. 2018;18(1). https://www.embase.com/search/results?subaction=viewrecord&id=L620583565&from=export.
Zhang D, He Z, Xu L, Zhu X, Wu J, Wen W, et al. Epidemiology characteristics of respiratory viruses found in children and adults with respiratory tract infections in southern China. Int J Infect Dis. 2014;25(((Zhang D.; He Z.) Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-sen University, Guangzhou, China):159–64.
Noh JY, Song JY, Cheong HJ, Choi WS, Lee J, Lee JS, et al. Laboratory Surveillance of Influenza-Like Illness in Seven Teaching Hospitals, South Korea: 2011–2012 Season. PLoS ONE [Internet]. 2013;8(5). https://www.embase.com/search/results?subaction=viewrecord&id=L368973555&from=export.
Ikematsu H, Takeuchi Y, Rosenlund M, Kawai N, Shimamura R, Hirata M, et al. The post-infection outcomes of influenza and acute respiratory infection in patients above 50years of age in Japan: an observational study. Influenza Other Respir Viruses. 2012;6(3):211–7.
Park E, Park PH, Huh JW, Yun HJ, Lee HK, Yoon MH, et al. Molecular and clinical characterization of human respiratory syncytial virus in South Korea between 2009 and 2014. Epidemiol Infect. 2017;145(15):3226–42.
Shih HI, Wang HC, Su IJ, Hsu HC, Wang JR, Sun HFS, et al. Viral Respiratory Tract Infections in Adult Patients Attending Outpatient and Emergency Departments, Taiwan, 2012–2013: A PCR/Electrospray Ionization Mass Spectrometry Study. Medicine (Baltimore). 2015;94(38): e1545.
Lu Y, Tong J, Pei F, Yang Y, Xu D, Ji M, et al. Viral aetiology in adults with acute upper respiratory tract infection in Jinan, Northern China. Clin Dev Immunol [Internet]. 2013;2013. https://www.embase.com/search/results?subaction=viewrecord&id=L368865637&from=export.
Kim JM, Jung HD, Cheong HM, Lee A, Lee NJ, Chu H, et al. Nation-wide surveillance of human acute respiratory virus infections between 2013 and 2015 in Korea. J Med Virol. 2018;90(7):1177–83.
Price OH, Sullivan SG, Sutterby C, Druce J, Carville KS. Using routine testing data to understand circulation patterns of influenza A, respiratory syncytial virus and other respiratory viruses in Victoria, Australia. Epidemiol Infect [Internet]. 2019 ed [cited 2020 Nov 25];147. https://www.cambridge.org/core/journals/epidemiology-and-infection/article/using-routine-testing-data-to-understand-circulation-patterns-of-influenza-a-respiratory-syncytial-virus-and-other-respiratory-viruses-in-victoria-australia/14B9D0E61E0E8AC3F0A3B8BB8B397198.
Xiang Z, Gonzalez R, Ren L, Xiao Y, Chen L, Zhang J, et al. Prevalence and clinical characteristics of human respiratory syncytial virus in Chinese adults with acute respiratory tract infection. J Med Virol. 2013;85(2):348–53.
Liao H, Yang Z, Yang C, Tang Y, Liu S, Guan W, et al. Impact of viral infection on acute exacerbation of asthma in out-patient clinics: a prospective study. J Thorac Dis. 2016;8(3):505–12.
Lee N, Lui GCY, Wong KT, Li TCM, Tse ECM, Chan JYC, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013;57(8):1069–77.
Zhang Y, Wang Y, Zhao J, Xiong Z, Fan Y, Zhang W, et al. Severity and mortality of respiratory syncytial virus vs influenza A infection in hospitalized adults in China. Influenza Other Respir Viruses. 2020;14(5):483–90.
Yang L, Hung Chan K, Suen LKP, Pan Chan K, Wang X, Cao P, et al. Age-specific epidemic waves of influenza and respiratory syncytial virus in a subtropical city. Sci Rep. 2015;5(1):10390.
Takahashi H, Jingu D, Yajima G, Ikuji S, Shoji S, Watanabe A. 当院において冬季 2 シーズンに経験した成人 RS ウイルス感染症例の臨床像 (The clinical image of respiratory syncytial virus infected adults who have been through two winter seasons at our hospital).pdf. J Infect Dis. 2016.
Saraya T, Kimura H, Kurai D, Ishii H, Takizawa H. The molecular epidemiology of respiratory viruses associated with asthma attacks. Med U S [Internet]. 2017;96(42). https://www.embase.com/search/results?subaction=viewrecord&id=L619281443&from=export.
Kwon YS, Park SH, Kim MA, Kim HJ, Park JS, Lee MY, et al. Risk of mortality associated with respiratory syncytial virus and influenza infection in adults. BMC Infect Dis [Internet]. 2017;17(1). https://www.embase.com/search/results?subaction=viewrecord&id=L619820465&from=export.
Yoon JG, Noh JY, Choi WS, Park JJ, Suh YB, Song JY, et al. Clinical characteristics and disease burden of respiratory syncytial virus infection among hospitalized adults. Sci Rep. 2020;10(1):12106.
Kwak HJ, Park DW, Kim JE, Park MK, Koo GW, Park TS, et al. Prevalence and risk factors of respiratory viral infections in exacerbations of chronic obstructive pulmonary disease. Tohoku J Exp Med. 2016;240(2):131–9.
Seo KH, Bae DJ, Kim JN, Lee HS, Kim YH, Park JS, et al. Prevalence of respiratory viral infections in Korean adult asthmatics with acute exacerbations: Comparison with those with stable state. Allergy Asthma Immunol Res. 2017;9(6):491–8.
Saravanos GL, Sheel M, Homaira N, Dey A, Brown E, Wang H, et al. Respiratory syncytial virus-associated hospitalisations in Australia, 2006–2015. Med J Aust. 2019;210(10):447–53.
Yin T, Zhu Z, Mei Z, Feng J, Zhang W, He Y, et al. Analysis of viral infection and biomarkers in patients with acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2018;12(3):1228–39.
Chadha M, Hirve S, Bancej C, Barr I, Baumeister E, Caetano B, et al. Human respiratory syncytial virus and influenza seasonality patterns—early findings from the WHO global respiratory syncytial virus surveillance. Influenza Other Respir Viruses [Internet]. 2020;((Chadha M.) National Institute of Virology, Indian Council of Medical Research, Pune, India). https://www.embase.com/search/results?subaction=viewrecord&id=L2004406386&from=export.
Lin WH, Chiu HC, Chen KF, Tsao KC, Chen YY, Li TH, et al. Molecular detection of respiratory pathogens in community-acquired pneumonia involving adults. J Microbiol Immunol Infect [Internet]. 2021 Dec 16 [cited 2022 Jan 6]; https://www.sciencedirect.com/science/article/pii/S168411822100270X.
He Y, Zhang J, Feng J, Zhoufang Z, Qian L, Huang Q, et al. 成人社区获得性肺炎合并呼吸道病毒感染住院患者病毒谱及临床特征分析. 中国全科医学. 24(26):3323–9.
Kurai D, Saraya T, Ishii H, Takizawa H. Virus-induced exacerbations in asthma and COPD. Front Microbiol [Internet]. 2013. https://doi.org/10.3389/fmicb.2013.00293/full.
Britto CJ, Brady V, Lee S, Dela Cruz CS. Respiratory viral infections in chronic lung diseases. Clin Chest Med. 2017;38(1):87–96.
RKI - Homepage [Internet]. [cited 2022 Jan 14]. https://www.rki.de/EN/Home/homepage_node.html;jsessionid=27073455ADCC6483546B0AE5374ED128.internet102.
Health protection: Infectious diseases - detailed information - GOV.UK [Internet]. [cited 2022 Jan 14]. https://www.gov.uk/topic/health-protection/infectious-diseases.
Homepage | European Centre for Disease Prevention and Control [Internet]. [cited 2022 Jan 14]. https://www.ecdc.europa.eu/en.
Acknowledgements
Funding
This study was sponsored and fully funded, including the journal’s Rapid Service Fee, by Janssen Pharmaceuticals.
Medical Writing and Editorial Assistance
Amish Chaturvedi, an employee of Janssen, provided editorial support. Vanessa Lacuesta and Jun Feng, employees of Janssen, provided valuable review and feedback.
Authors Contributions
Conceptualization and design: Luis Hernandez-Pastor, Tom Hsun-Wei Huang, SeongBeom Park, KyungHwa Lim, Petar Atanasov; Research conduction and data analysis: Xiaobin Jiang, Petar Atanasov; Data interpretation: Daisuke Kurai, Joon Young Song, Yhu-Chering Huang, Zhijun Jie, Luis Hernandez-Pastor, Tom Hsun-Wei Huang, SeongBeom Park, KyungHwa Lim, Peter Richmond; Manuscript writing: Xiaobin Jiang, Petar Atanasov; Manuscript review and editing: Daisuke Kurai, Joon Young Song, Yhu-Chering Huang, Zhijun Jie, Luis Hernandez-Pastor, Tom Hsun-Wei Huang, SeongBeom Park, KyungHwa Lim, Peter Richmond.
Disclosures
Luis Hernandez-Pastor, Tom Hsun-Wei Huang, SeongBeom Park, and KyungHwa Lim were employees from Janssen Pharmaceuticals at the time of conduction of this study. Xiaobin Jiang and Petar Atanasov were employees of Amaris Consulting, a paid consultant to Janssen Pharmaceuticals to support the conduction of this study. Peter Richmond has served on scientific advisory boards for RSV vaccines and monoclonal antibodies on behalf on his institution for Janssen, GSK, Merck, Sanofi, Pfizer and Astra-Zeneca.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kurai, D., Song, J., Huang, YC. et al. Targeted Literature Review of the Burden of Respiratory Syncytial Infection among High-Risk and Elderly Patients in Asia Pacific Region. Infect Dis Ther 12, 807–828 (2023). https://doi.org/10.1007/s40121-023-00777-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00777-2