Abstract
Introduction
Antiretroviral therapy (ART) has been associated with weight gain in people living with HIV-1 (PLWH); however, limited research has assessed whether early weight gain post-ART initiation is associated with metabolic or cardiovascular outcomes among PLWH at high risk of weight gain (i.e., female, Black or Hispanic). This study aimed to evaluate the incidence of metabolic and cardiovascular outcomes between PLWH at high risk of weight gain following an observed ≥ 5% or < 5% weight/body mass index (BMI) gain within 6 months following ART initiation.
Methods
A retrospective longitudinal study using Symphony Health, an ICON plc Company, IDV® electronic medical records (October 1, 2014–March 31, 2021) identified adult female, Black, or Hispanic treatment-naïve PLWH who initiated ART and who had ≥ 1 weight or BMI measurement pre- and within 6 months post-treatment (landmark period). Inverse probability of treatment weighting was used to account for differences between PLWH who experienced ≥ 5% and < 5% weight/BMI gain. The time to each outcome was compared between cohorts using weighted hazard ratios (HRs) after the landmark period.
Results
Weighted ≥ 5% and < 5% cohorts included 620 and 632 patients, respectively; baseline characteristics were similar between the two cohorts (mean age: ~ 48 years, ~ 59% female, ~ 49% Black, ~ 17% Hispanic). During a mean 2-year follow-up, PLWH with ≥ 5% weight/BMI gain were significantly more likely to be diagnosed with type 2 diabetes mellitus (T2DM; HR = 2.19; p = 0.044). There were no significant differences in the incidence of any other outcomes between the study cohorts.
Conclusion
Despite a short 2-year follow-up, female, Black or Hispanic PLWH experiencing ≥ 5% weight/BMI increase within 6 months following ART initiation had an increased risk of T2DM, but not other metabolic or cardiovascular outcomes, likely due to the short follow-up period. Further research with longer follow-up and specific ART regimens is warranted to examine the impact of ART-related weight gain on long-term clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Initiation of antiretroviral therapy (ART) greatly improves HIV-1 outcomes, transmission and quality of life, but has also been associated with weight gain, particularly in female, Black or Hispanic people living with HIV-1 (PLWH). |
This study sought to identify the association of ART-associated weight gain (≥ 5% versus < 5% weight/BMI gain within 6 months post-ART initiation) with incident metabolic and cardiovascular outcomes among PLWH at risk of weight gain. |
What was learned from this study? |
During a mean 2-year follow-up, PLWH with ≥ 5% ART-associated weight gain or BMI increase within 6 months of ART initiation were significantly and substantially more likely to be diagnosed with type 2 diabetes mellitus (T2DM; HR = 2.19; p = 0.044), but not with other metabolic or cardiovascular events, likely due to short follow-up. |
These findings provide evidence bridging the link between ART-associated weight gain and incident T2DM in PLWH at high risk of weight gain; further studies with longer follow-up may help further elucidate the impact of ART-associated weight gain on cardiovascular and metabolic diseases in these patients. |
Introduction
Background/Rationale
HIV-1 is a chronic infectious disease which, while incurable, can be effectively managed with the use of antiretroviral therapy (ART). ART effectively reduces the risk of transmission [1,2,3,4,5] and improves clinical outcomes [6] and quality of life [3] in people living with HIV-1 (PLWH). There are several classes of ARTs, including integrase strand transfer inhibitor (INSTI)-based, protease inhibitor (PI)-based and non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART regimens, with the US Department of Health and Human Services (DHHS) guidelines currently recommending use of INSTI-based regimens in most clinical situations [7].
While all three ART classes are associated with weight gain in a real-world context to varying extents, DHHS guidelines as well as several studies have reported that INSTI-based regimens are associated with greater weight gain than PI- or NNRTI-based ART regimens in treatment-naïve PLWH [7,8,9]. Prior evidence also suggest that weight gain has been observed in the year following initiation of ART [10, 11], sometimes as early as 3 months after treatment initiation [9]. ART-related weight gain also does not affect all PLWH equally, as certain demographic groups, including female, Black and Hispanic PLWH, are generally at greater risk of ART-related weight gain [10, 12, 13], particularly following initiation of INSTI-based regimens [14].
As life expectancy of PLWH increases, other health outcomes outside of HIV-1 infection are becoming more prevalent in this population [15]. Given the known link between weight gain and numerous health conditions including metabolic and cardiovascular outcomes in the general population [16, 17], it is important to understand the impact of early ART-related weight gain on incidence of cardiometabolic diseases. Furthermore, it is important to contextualize this relationship particularly among PLWH at greater risk of ART-related weight gain, given that cardiometabolic disorders such as obesity and diabetes in particular pose a significant burden to Black and Hispanic populations regardless of HIV status [18, 19]. In these populations, the higher risk of incident diabetes may be related to diet, lack of access to health care and other socio-economic determinates of health, which disproportionally affect Black and other minority PLWH [20, 21].
There is a growing body of research investigating the impact of ART-associated weight gain on diseases where weight gain is implicated. The use of specific ART regimens and resulting weight gain has been associated with incident diabetes [12, 22], and certain factors such as HIV viremia and obesity have been associated with developing type 2 diabetes mellitus (T2DM) [23]. It has also been shown that among PLWH, Black race or Hispanic ethnicity is a major risk factor for incident diabetes following ART treatment [22, 24], though research is sparse on females. Although HIV-1 infection is associated with a roughly twofold risk of atherosclerotic disease [25], there are relatively few studies that have assessed the risk of metabolic, cardiovascular and cardiometabolic outcomes at a large scale. In particular, studies that assessed association between weight gain and cardiometabolic outcomes did not focus on the consequences of early weight gain observed within 6 months after ART initiation [26]. Therefore, understanding the implications of early ART-associated weight gain on metabolic and cardiovascular outcomes is critical when choosing an initial ART regimen for PLWH at high risk of weight gain.
Objectives
This study aims to compare the incidence of metabolic (i.e., T2DM, hypertension, lipid disorders, lipodystrophy and metabolic syndrome) and cardiovascular conditions (i.e., myocardial infarction, congestive heart failure, coronary artery disease and stroke/transient ischemic attack) among PLWH at high risk of weight gain who experience early weight gain or BMI increase ≥ 5% within 6 months following ART initiation compared to those with weight gain or BMI increase < 5%.
Methods
Data Source
Administrative claims and electronic medical record (EMR) data (October 1, 2014–March 31,2021) from the Symphony Health, an ICON plc Company, IDV® database were used to identify the population and all variables of interest and to conduct the analysis. The IDV® database links healthcare data for the US population from three basic sources: pharmacy point-of-service, switch/network transactions and additional direct prescription, medical and hospital claims data. This provider-based data source includes claimant demographics, medical and procedure claims, as well as historical clinical information from linked EMR data capturing diagnoses, medications prescribed and administered, laboratory results and vital signs including BMI, height and weight measurements. The data were de-identified and compliant with the patient information requirements of the Health Insurance Portability and Accountability Act (HIPAA); therefore, no review by an institutional review board was required per Title 45 of CFR, Part 46.101(b)[4].
Study Design
A retrospective longitudinal cohort study design was used for this analysis. Adult female, Black or Hispanic PLWH who were treatment-naïve and initiated their first complete DHHS-recommended ART regimen were identified in the EMR (see Supplementary Material Table 1 for a complete list of DHHS-recommended ART regimens). The date of initiation of the PI, INSTI or NNRTI agent, which was part of the ART regimen, was defined as the index date. For single-tablet regimens (STRs), the index date was the date of the first prescription of the STR, and for multiple-tablet regimens (MTRs), the index date was defined as the date of the first prescription for the PI, INSTI or NNRTI agent received as part of a complete ART regimen containing ≥ 2 nucleoside reverse transcriptase inhibitors (NRTIs) or as part of an NRTI-sparing regimen (i.e., dolutegravir + rilpivirine or darunavir/cobicistat + raltegravir), with all agents being received within 14 days before or after the index date.
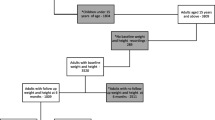
Periods of continuous clinical activity in the EMR data were defined as the period from the first to the last interaction within the database. To ensure all PLWH were treatment naïve, a 12-month baseline period without any PI, INSTI or NNRTI agents was required prior to the index date (i.e., washout period). The baseline period was followed by a 6-month landmark period where BMI and weight changes from baseline were assessed to identify the cohorts of interest. The observation period to evaluate cardiometabolic outcomes started after the 6-month landmark period and ended at the end of continuous clinical activity or end of data availability (i.e., March 31, 2021), whichever occurred first. The study design is depicted in Fig. 1.
Study design. ART antiretroviral therapy, BMI body mass index, DHHS Department of Health and Human Services, INSTI integrase strand transfer inhibitor, NNRTI non-nucleoside reverse transcriptase inhibitor, PI protease inhibitor. aBaseline laboratory and weight/BMI values were obtained from the baseline period measurement prior to and closest to the index date. bAll weight/BMI values observed during the landmark period were assessed to determine whether there was a weight/BMI gain ≥ 5% relative to the baseline value
Study Population and Study Size
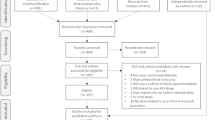
Patients were assigned to a cohort based on whether they had weight gain or BMI increase ≥ 5% between the baseline period and the landmark period. The closest weight/BMI measure prior to the index date was considered as the baseline weight/BMI. All weight/BMI measures during the landmark period were considered to evaluate change in weight/BMI; a patient with ≥ 1 weight/BMI measurement during the landmark period indicating a gain of ≥ 5% was considered part of the ≥ 5% cohort and patients with all weight/BMI measurements during the landmark period indicating < 5% gain were assigned to the < 5% cohort. The sample selection is depicted in Fig. 2.
Adult female (based on sex at birth), Black or Hispanic PLWH who initiated their first complete DHHS-recommended ART regimen were eligible to be included in the study if they met the following study criteria: initiated a PI, INSTI or NNRTI-based ART regimen, had ≥ 1 diagnosis of HIV-1 on or before the index date, ≥ 12 months of continuous clinical activity before the index date (baseline period), ≥ 6 months of continuous clinical activity after the index date (landmark period) and ≥ 1 weight measurement in both the baseline and landmark periods or ≥ 1 BMI measurement in both the baseline and landmark periods (Fig. 2).
PLWH were excluded if they met any of the following criteria: had ≥ 1 written prescription for a PI, INSTI or NNRTI agent during the baseline period or ≥ 1 diagnosis for one of the following during the baseline period: HIV-2, liver disease (including cirrhosis and hepatitis), stage V chronic kidney disease or end-stage renal disease (or creatinine clearance < 15 ml/min), pregnancy and cancer (excluding cutaneous Kaposi’s sarcoma, basal cell carcinoma or resected, non-invasive cutaneous squamous carcinoma) (Fig. 2).
Variables
Demographic and clinical characteristics of the study population were evaluated during the 12-month baseline period, and clinical characteristics were also evaluated during the 6-month landmark period. Incident metabolic or cardiovascular outcomes were evaluated separately during the observation period. The metabolic outcomes included T2DM, hypertension, lipid disorders, lipodystrophy and metabolic syndrome. Cardiovascular outcomes included coronary artery disease, congestive heart failure, stroke and myocardial infarction. A composite cardiometabolic outcome encompassing any one of these conditions was also included (see Supplementary Material Table 2 for a list of diagnosis codes and proxy medication classes for metabolic and cardiovascular outcomes). To confirm incident metabolic or cardiovascular outcomes, ≥ 2 diagnoses using the EMR or claims data were required during the observation period. PLWH were eligible for the analysis of each outcome for which they were at risk during the observation period (i.e., did not have the metabolic or cardiovascular condition during the baseline or landmark periods). Presence of the condition during the baseline or landmark periods was defined as either ≥ 1 diagnosis for the condition in EMR or claims data or ≥ 1 written prescription for a proxy medication suggestive of the condition in EMR data (e.g., antidiabetics for T2DM; Supplementary Material Table 2). For the composite cardiometabolic outcomes, the analysis included all patients; however, only incident conditions were considered in the composite outcome. In other words, patients with the presence of a specific condition during the baseline or landmark periods could not develop that same condition during the observation period, but they could develop another cardiometabolic outcome that they did not have during the baseline or landmark periods.
Statistical Methods
Baseline and landmark demographic and clinical characteristics, the length of the observation period, the number of PLWH at risk for a given outcome and the number of incident outcomes during the observation period (reported separately for each outcome) were reported using means, standard deviations (SDs) and medians for continuous variables, and frequencies and proportions for categorical variables. Missing data were accounted for by reporting proportions among patients with information of a given variable or by reporting the proportion of patients with unknown data, where applicable.
To isolate the effect of weight/BMI increase on the incidence of metabolic or cardiovascular outcomes and to minimize possible bias due to confounding variables between the two cohorts, patients in the ≥ 5% and < 5% cohorts were weighted based on inverse probability of treatment weighting (IPTW). These weights were based on propensity scores (PS), which were generated using a logistic regression model in which the dependent variable was weight/BMI increase (≥ 5% or < 5%) and baseline demographic and clinical characteristics and index ART regimen were independent variables used to predict treatment assignment. The following baseline variables were included in the PS model: age, sex, race, geographic region, insurance plan type, year of index date, Quan-Charlson Comorbidity index (Quan-CCI; excluding HIV-1 symptoms), weight loss, weight gain medications, weight loss medications, antihypertensives, antihyperlipidemics, antidiabetics, time between HIV-1 onset and the index date, and use of a PI, INSTI, NNRTI, tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF) as part of the index regimen.
IPTW weighting was also carried out for a subgroup of the study population who had BMI ≥ 25 kg/m2 during the baseline period. The following baseline variables were included in this PS model: age, sex, race, geographic region, insurance plan type, year of index date, Quan-Charlson Comorbidity index (excluding HIV-1 symptoms), weight loss, weight gain medications, weight loss medications and use of a PI, INSTI, NNRTI, TDF and TAF as part of the index regimen.
IPTW weights for each patient were estimated as follows: 1/PS for patients in the ≥ 5% cohort and 1/(1 − PS) for patients in the < 5% cohort. Weights were normalized by the mean weight and trimmed to the 95th percentile. After weighting, baseline and landmark period characteristics were compared and standardized differences of < 10% were considered balanced [27]. While the same number of patients contributed to the analysis, the resulting weighted sample sizes from this procedure were different from the original cohort sample sizes attributed to the fact that each patient was assigned a different weight after IPTW than the original sample.
The number of incident events for each individual metabolic and cardiovascular outcome was assessed in the overall cohort and the subgroup of patients with BMI ≥ 25 kg/m2. Results were reported as the incidence rate per thousand patient years (PTPY), calculated by dividing the number of observed incident events by the total follow-up time available. Follow-up time was censored at the incident event, if it was observed. Weighted Cox proportional hazards models were used to generate hazard ratios (HRs) comparing the time to each individual incident cardiometabolic outcome between the ≥ 5% and < 5% cohort. To account for the variability in individuals’ IPTW, 95% confidence intervals (CIs) and p-values were generated using non-parametric bootstrap procedures with 500 resamples.
Results
Overall, 1252 PLWH at high risk of weight gain were eligible for inclusion in the study (Fig. 2). Of these, 366 (29.2%) experienced weight gain or BMI increase of ≥ 5%, while 886 (70.8%) experienced weight gain or BMI increase of < 5%. After applying IPTW, weighted sample sizes were 620 and 632 patients in the ≥ 5% and < 5% cohorts, respectively (Table 1). For the BMI ≥ 25 kg/m2 subgroup, 780 PLWH at high risk of weight gain were eligible, including 228 patients and 552 patients with weight gain/BMI increase ≥ 5% and < 5%, respectively. After applying IPTW, weighted sample sizes were 384 and 396 patients in the ≥ 5% and < 5% cohorts, respectively (Supplementary Table 3).
Baseline and Landmark Characteristics
Baseline and landmark characteristics for the weighted cohorts are presented in Table 1. The mean age was 47.8 (SD = 13.0) years in both cohorts, with a similar proportion of females (≥ 5% cohort: 59.9%; < 5% cohort: 58.2%), Blacks (≥ 5% cohort: 49.2%; < 5% cohort: 49.3%) and Hispanics (≥ 5% cohort: 16.3%; < 5% cohort: 17.2%) in each cohort. Most patients resided in the South (68.9% in both cohorts) and were covered exclusively by commercial insurance (≥ 5% cohort: 60.0%; < 5% cohort: 59.6%). The mean baseline Quan-CCI score (excluding HIV-1 symptoms) was 1.1 in both cohorts. Index regimens were similar between the cohorts, with most patients initiating an INSTI-based regimen (≥ 5% cohort: 78.0%; < 5% cohort: 77.1%). Nearly half of patients in each cohort initiated a regimen containing TAF (≥ 5% cohort: 44.8%; < 5% cohort: 43.2%). During the baseline period, a similar number of patients in each cohort were on medications associated with weight gain (≥ 5% cohort: 25.6%; < 5% cohort: 24.3%). Baseline characteristics were similarly well balanced for the BMI ≥ 25 kg/m2 subgroup (Supplementary Table 3).
The most common cardiometabolic comorbidities observed during the baseline period were hypertension (≥ 5% cohort: 48.6%; < 5% cohort: 44.7%) and lipid disorders (≥ 5% cohort: 36.4%; < 5% cohort: 32.8%). These were also the most common comorbidities observed during the landmark period. The mean weight during the baseline period, among patients with a measurement during both baseline and landmark periods, was 87.3 kg in the ≥ 5% cohort and 84.5 kg in the < 5% cohort. The mean weight change observed between the baseline value and the highest measured value during the landmark period was + 6.7 kg in the ≥ 5% cohort and –0.1 kg in the < 5% cohort (Table 2).
Incident Cardiometabolic Outcomes
Overall, a mean length of 2.0 (SD ± 1.3) years of follow-up was observed in both cohorts. Evaluation of the associations between weight/BMI gain of ≥ 5% or < 5% and incidence of each metabolic and cardiovascular outcome of interest are presented in Fig. 3.
Incident cardiometabolic outcomes comparison. BMI body mass index, CI confidence interval, HR hazard ratio, ICD-9-CM/ICD-10-CM International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification, PLWH people living with HIV-1, PTPY per thousand patient-years. *Significant at the 5% level. aOf note, the number of patients reported in this weighted population represents the sum of weights for the corresponding patients, rounded to the nearest integer. The proportions displayed were calculated prior to the rounding and may be slightly different than if they were calculated based on rounded numbers. bType 2 diabetes, hypertension, lipid disorders, lipodystrophy, metabolic syndrome, coronary artery disease, congestive heart failure, stroke/transient ischemic attack or myocardial infarction. cThe incidence of metabolic syndrome may have been underestimated in the current analysis, as it was identified solely based on ICD-9-CM/ICD-10-CM codes and information such as waist circumference was not available. dIt was not possible to evaluate the hazard ratio for these outcomes because there were no incident events for at least one cohort
PLWH in the ≥ 5% cohort were substantially and significantly more likely to experience incident T2DM (HR = 2.19; p = 0.044) compared to PLWH in the < 5% cohort; incidence rates for T2DM were 31.2 events PTPY for the ≥ 5% cohort and 13.7 events PTPY for the < 5% cohort.
Incidence rates for the composite metabolic outcome were 436.4 PTPY in the ≥ 5% cohort and 399.8 PTPY in the < 5% cohort, although this result did not attain statistical significance (HR = 1.09; p = 0.248). There were no significant differences in the incidence of other specific cardiovascular or metabolic outcomes between the two study cohorts, with HRs ranging from 0.44 (p = 0.220) for congestive heart failure to 1.07 (p = 0.820) for lipid disorders. Due to a small number of events in both cohorts, HRs were not evaluable for metabolic syndrome and myocardial infarction (Fig. 3). As presented in Supplementary Fig. 1, results for the BMI ≥ 25 kg/m2 subgroup were consistent with overall cohort results but not significant.
Discussion
Key Results
This retrospective, longitudinal study used EMR and claims data to identify treatment-naïve PLWH who are at high risk of weight gain (i.e., female, Black or Hispanic) and generated well-balanced cohorts of PLWH who experienced ≥ 5% or < 5% weight gain or BMI increase within 6 months following ART initiation. Over a mean follow-up of 2 years, PLWH who experienced ≥ 5% weight gain or BMI increase were more than two times more likely to be diagnosed with incident T2DM than PLWH who experienced < 5% weight gain or BMI increase. This is consistent with other research among treatment-naïve PLWH initiating ART and provides additional context by confirming these results in PLWH at high risk of weight gain.
Interpretation
An increasing large body of literature has assessed risk factors for T2DM among PLWH [28, 29]. More specifically, one study has found that a unit of BMI increase at 1 year was associated with an increased risk of T2DM among PLWH with normal BMI at ART initiation (incidence rate ratio [IRR]: 1.11) [11]. In addition, a recent study has reported that initiating INSTI-based ART regimens, which have been associated with more weight gain than PI- and NNRTI-based treatment regimens, was associated with more incident T2DM diagnoses compared to non-INSTI regimens (HR = 3.27; p = 0.014) [12]. While the aforementioned studies have looked specifically at T2DM risk, many studies have assessed purely ART-associated weight change. As an example, a recent study showed that ART was associated with weight and BMI gain after initiating treatment, along with a significantly greater mean weight gain (1.5 kg) in Black female PLWH who initiated an INSTI compared to a PI over a mean follow-up of 9.5 months [30]. Findings in Hispanic PLWH also trended in the same direction [30]. The importance of the results of this current study is that they bridge the gap between research like the one just mentioned above, which is investigating ART and weight gain, by isolating the impact of ART-associated weight gain on cardiometabolic outcomes. This provides findings which can be considered for female, Black or Hispanic PLWH who are initiating ART, although additional research on the clinical significance of the early weight gain after ART initiation is needed.
Another finding of the study is the lack of statistical significance in the other metabolic and cardiovascular outcomes of interest in the overall cohort as well as in the subgroup of patients with BMI ≥ 25 kg/m2, as low numbers (i.e., < 10 events per year) were observed for some metabolic and nearly all cardiovascular outcomes including coronary artery disease, congestive heart failure and myocardial infarction. Prior research evaluating the risk of cardiovascular outcomes following BMI gain after ART initiation, over a follow-up period of 5 years post-ART initiation, reported a significant increased risk of cardiovascular disease (IRR: ~ 1.2), but only among PLWH having normal BMI pre-ART initiation [11]. This suggests that further studies with a longer follow-up period would be useful to continue to elucidate the long-term impact of ART-associated weight gain on the other cardiometabolic outcomes, particularly cardiovascular outcomes.
An important contribution of this study is the focus on PLWH at high risk of weight gain (i.e., female, Black or Hispanic PLWH). Black race and Hispanic ethnicity are associated with higher risk for T2DM independent of HIV-1 status, and previous research has demonstrated that HIV-1 infection is associated with an increased burden related to diabetes and cardiovascular disease [22, 25, 31]. Previous studies have also identified that female, Black or Hispanic PLWH have significantly greater weight gain when initiating ART [10, 32]. This is potentially important given the possible intersections of illness with other factors such as gender identification, diet, socioeconomic status, access to healthcare and other social determinants of health [33], not captured by this study, but which may also influence weight gain and thus disease risk. The results of this study suggest that for T2DM, ART-associated weight gain may be an additional risk factor to consider when evaluating disease risk. The results of this study link weight gain within 6 months of ART initiation to incident T2DM in this patient population, suggesting a need to consider these outcomes for female, Black or Hispanic PLWH newly initiating ART.
Limitations
The findings of this study should be interpreted considering certain limitations. First, the impact of specific ART classes or regimens on weight gain and cardiometabolic outcomes was not assessed and therefore the results do not necessarily apply equally to all ARTs. In addition, since the study population included PLWH at high risk of weight gain who were treatment-naïve, results may not be generalizable to males or white populations; however we focused on these populations with the knowledge that most of the existing data include white males. Second, PLWH, particularly Black or Hispanic PLWH, can gain weight for reasons other than ART use; for instance, information on lifestyle measures, socioeconomic status or family/social history may have an impact but were not available in the data. Similarly, laboratory testing data including CD4+/CD8+ cell count measurements, HIV-1 viral load, cholesterol and lipid panel data were sparse in the data and thus were not included in the analysis. Therefore, the results are demonstrative of associations and should not be interpreted as causal. Third, information on waist circumference was lacking in the EMR data and thus the complete impact of ART-related adiposity on metabolic and cardiovascular outcomes could not be assessed; this may specifically have impacted the assessment of metabolic syndrome. Fourth, imposing 6 months of clinical activity after the index date (i.e., landmark period) may have resulted in survival bias and the selection of more healthy patients. Fifth, the definition of treatment-naïve employed in this study required a 12-month period prior to ART initiation without any PI, INSTI or NNRTI use, but this may include some PLWH who had ART exposure > 12 months prior and then re-initiated ART at the index date. Sixth, despite small imbalances in baseline weight and BMI, these were not included in the PS model because they were directly related to the exposure definition of ≥ 5% weight or BMI increase between the baseline and landmark periods. Seventh, only information pertaining to sex at birth was available, and while it was observed that approximately 1.5% of patients received hormonal therapy, it was not possible to assess the impact of weight gain or BMI increase among transgender PLWH. Lastly, this study had a relatively short follow-up period, which may have explained the lack of association with some of the outcomes. In particular, since cardiovascular outcomes may develop over longer periods of time, the short-term follow-up may have impacted the ability of the study to capture them. However, the short follow-up period makes our finding of the association between weight gain and T2DM even more notable.
Conclusion
Despite a short follow-up of 2 years, female, Black or Hispanic PLWH experiencing ≥ 5% weight/BMI increase within 6 months following initiation of ART had more than doubled their risk of incident T2DM. There were no other significant differences in metabolic or cardiovascular outcomes observed, which is likely due to the short follow-up period. Further research with longer follow-up is warranted to examine the impact of ART-related weight gain on long-term clinical outcomes among PLWH, but these data suggest that, at least for T2DM, the implications of early weight gain after ART initiation are significant and should not be merely seen as a positive “return-to-health” phenomenon.
References
Bavinton BR, Pinto AN, Phanuphak N, Grinsztejn B, Prestage GP, Zablotska-Manos IB, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018;5(8):e438–47.
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505.
Farnham PG, Gopalappa C, Sansom SL, Hutchinson AB, Brooks JT, Weidle PJ, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr. 2013;64(2):183–9.
Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–9.
Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner Is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–81.
Fischl MA, Richman DD, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317(4):185–91.
U.S. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV 2021. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/whats-new-guidelines.
Bourgi K, Jenkins CA, Rebeiro PF, Shepherd BE, Palella F, Moore RD, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23(4): e25484.
Chow W, Donga P, Côté-Sergent A, Rossi C, Lefebvre P, Lafeuille M-H, et al. An assessment of weight change associated with the initiation of a protease or integrase strand transfer inhibitor in patients with human immunodeficiency virus. Curr Med Res Opin. 2020;36(8):1313–23.
Sax PE, Erlandson KM, Lake JE, Mccomsey GA, Orkin C, Esser S, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2019;71(6):1379–89.
Achhra AC, Mocroft A, Reiss P, Sabin C, Ryom L, de Wit S, et al. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A: D study. HIV Med. 2016;17(4):255–68.
Asundi A, Olson A, Jiang W, Varshney SP, White LF, Sagar M, et al. Integrase inhibitor use associated with weight gain in women and incident diabetes mellitus. AIDS Res Hum Retroviruses. 2022;38(3):208–15.
Bedimo R, Adams-Huet B, Taylor BS, Lake J, Luque A. 538. Integrase inhibitor-based HAART is associated with greater BMI gains in Blacks, Hispanics, and women. Open Forum Infect Dis. 2018;5(S1):S199.
Chen YW, Hardy H, Pericone CD, Chow W. Real-world assessment of weight change in people with HIV-1 after initiating integrase strand transfer inhibitors or protease inhibitors. J Health Econ Outcomes Res. 2020;7(2):102–10.
Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities among US patients with prevalent HIV infection-A trend analysis. J Infect Dis. 2017;216(12):1525–33.
Bae YS, Choi S, Lee K, Son JS, Lee H, Cho MH, et al. Association of concurrent changes in metabolic health and weight on cardiovascular disease risk: a nationally representative cohort study. J Am Heart Assoc. 2019;8(17): e011825.
Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care. 2007;30(6):1562–6.
Centers for Disease Control. BY the numbers: diabetes in America 2022. https://www.cdc.gov/diabetes/health-equity/diabetes-by-the-numbers.html.
Petersen R, Pan L, Blanck HM. Racial and ethnic disparities in adult obesity in the United States: CDC’s tracking to inform state and local action. Prev Chronic Dis. 2019;16:E46.
Galaviz KI, Varughese R, Agan BK, Marconi VC, Chu X, Won SH, et al. The intersection of HIV, diabetes, and race: exploring disparities in diabetes care among people living with HIV. J Int Assoc Provid AIDS Care. 2020;19:2325958220904241.
Hill-Briggs F, Adler NE, Berkowitz SA, Chin MH, Gary-Webb TL, Navas-Acien A, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2021;44(1):258–79.
Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Epidemiology. 2018;29(3):431–41.
Ye Y, Shrestha S, Burkholder G, Bansal A, Erdmann N, Wiener H, et al. Rates and correlates of incident type 2 diabetes mellitus among persons living with HIV-1 infection. Front Endocrinol (Lausanne). 2020;11: 555401.
Chinaeke EE, Li M, Bookstaver B, Love BL, Li X, Reeder G, et al. Management of infection among Medicare beneficiaries with HIV/AIDS: risk of diabetes with protease inhibitors and associated racial disparities using big data approach. AIDS Care. 2020;2020:1–9.
Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation. 2018;138(11):1100–12.
McCann K, Shah S, Hindley L, Hill A, Qavi A, Simmons B, et al. Implications of weight gain with newer anti-retrovirals: 10-year predictions of cardiovascular disease and diabetes. AIDS. 2021;35(10):1657–65.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107.
Duncan AD, Goff LM, Peters BS. Type 2 diabetes prevalence and its risk factors in HIV: a cross-sectional study. PLoS ONE. 2018;13(3): e0194199.
Bratt G, Brannstrom J, Missalidis C, Nystrom T. Development of type 2 diabetes and insulin resistance in people with HIV infection: prevalence, incidence and associated factors. PLoS ONE. 2021;16(6): e0254079.
Chen Y-W, Anderson D, Pericone CD, Donga P. Real-world assessment of weight change in African American females and Hispanics with HIV-1 after initiating integrase strand-transfer inhibitors or protease inhibitors. J Health Econ Outcomes Res. 2022;9(1):1–10.
Centers for Disease Control and Prevention. Diabetes risk factors 2021. https://www.cdc.gov/diabetes/basics/risk-factors.html.
Coelho LE, Jenkins CA, Shepherd BE, Pape JW, Cordero FM, Padgett D, et al. Weight gain post-ART in HIV+ Latinos/as differs in the USA, Haiti, and Latin America. Lancet Reg Health Am. 2022;8(100173):1–11.
Centers for Disease Control. Causes of obesity 2022. https://www.cdc.gov/obesity/basics/causes.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fobesity%2Fadult%2Fcauses.html.
Acknowledgements
Funding
This study was funded by Janssen Scientific Affairs, LLC. The study sponsor was involved in several aspects of the research, including the study design, the interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication. The study sponsor has also funded the journal’s Rapid Service Fees.
Medical writing assistance
Medical writing assistance was provided by Jonah Gorodensky and Flora Chik, employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
BE, AS, CR, KM, and M-HL contributed to study conception and design, collection and assembly of data, and data analysis and interpretation. GAM, BB, and PD contributed to study conception and design, data analysis and interpretation. All authors reviewed and approved the final content of this manuscript.
Prior presentation
This manuscript contains research that has been presented previously at the ACTHIV 2022 conference on May 5–7, 2022, in Denver, Colorado.
Disclosures
Grace A. McComsey is a consultant for Janssen Scientific Affairs LLC, but did not receive any compensation for the design, writing, or reviewing of this manuscript. Grace A. McComsey is also a scientific consultant for Gilead, ViiV, Theratechnologies, and Merck. Bruno Emond, Aditi Shah, Carmine Rossi, Katherine Milbers, and Marie-Hélène Lafeuille are employees of Analysis Group, Inc., a company that provided consulting services to Janssen Scientific Affairs, LLC for the development and conduct of this study and manuscript. Brahim K. Bookhart and Prina Donga are employees of Janssen Scientific Affairs, LLC and are stockholders of Johnson & Johnson.
Compliance with ethics guidelines
The data were de-identified and compliant with the patient information requirements of the Health Insurance Portability and Accountability Act (HIPAA); therefore, no review by an institutional review board was required per Title 45 of CFR, Part 46.101(b)[4].
Data availability
The data that support the findings of this study are available from Symphony Health, an ICON plc Company, but restrictions apply to the availability of these data, which were used pursuant to a data use agreement. The data are available through requests made directly to ICON, subject to ICON’s requirements for data access.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
McComsey, G.A., Emond, B., Shah, A. et al. Association Between Weight Gain and the Incidence of Cardiometabolic Conditions Among People Living with HIV-1 at High Risk of Weight Gain Initiated on Antiretroviral Therapy. Infect Dis Ther 11, 1883–1899 (2022). https://doi.org/10.1007/s40121-022-00673-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00673-1