Abstract
Introduction
Glecaprevir/pibrentasvir is approved for treating chronic hepatitis C virus (HCV) genotypes (GT) 1–6. We evaluated real-world effectiveness, safety, and patient-reported outcomes of glecaprevir/pibrentasvir in underserved patient populations, focusing on persons who use drugs infected with HCV.
Methods
Data were pooled from nine countries (13 November 2017–31 January 2020). Patients had HCV GT1–6, with or without compensated cirrhosis, with or without prior HCV treatment and received glecaprevir/pibrentasvir consistent with local label at their physician’s discretion. Patients with prior direct-acting antiviral exposure were excluded from efficacy and quality-of-life analyses. The percentage of patients achieving sustained virologic response at post-treatment week 12 (SVR12) was assessed. Mean changes from baseline to SVR12 visit in 36-Item Short-Form Health Survey mental and physical component summary scores were reported. Safety was assessed in patients receiving at least one dose of glecaprevir/pibrentasvir.
Results
Of 2036 patients, 1701 (83.5%) received 8-week glecaprevir/pibrentasvir. In 1684 patients with sufficient follow-up, SVR12 rates were 98.0% (1651/1684) overall, 98.1% (1432/1459) in 8-week treated patients, 97.0% (519/535) in persons who use drugs, and greater than 95% across subgroups. Mean changes from baseline in mental and physical component summary scores were 3.7 and 2.4, respectively. One glecaprevir/pibrentasvir-related serious adverse event was reported; six glecaprevir/pibrentasvir-related adverse events led to discontinuation.
Conclusions
Glecaprevir/pibrentasvir was highly effective, well tolerated, and improved quality of life in HCV-infected persons who use drugs and other underserved patients.
Trial Registration
These multinational post-marketing observational studies are registered with ClinicalTrials.gov, number NCT03303599.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The global elimination of HCV infection is a possibility with the availability of short-course pangenotypic direct-acting antivirals, however rapid expansion of treatment to historically marginalized patients is necessary to achieve the goal of elimination. |
This multinational study presents the efficacy, safety, and patient-reported outcomes of treatment with G/P in historically underserved patient populations in real-world settings. |
What was learned from the study? |
Overall, G/P treatment was highly effective with a cure rate of 98% and well-tolerated across a broad range of marginalized patients with chronic HCV and single or dual comorbidities. |
Results from this real-world analysis address current gaps in clinical knowledge regarding impact on quality of life and G/P treatment led to improvements in health-related quality of life and reductions in fatigue. |
These results further support the use of an 8-week short-course G/P in underserved patients including those with substance abuse and psychiatric comorbidities. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14541573.
Introduction
Interferon-free pangenotypic direct-acting antivirals (DAA) have transformed the treatment landscape for patients with hepatitis C virus (HCV) infection [1,2,3]. Their introduction has shifted the focus of HCV management from meeting specific unmet medical needs of patients to achieving the overarching goal of HCV elimination. The World Health Organization (WHO) has set targets for eliminating HCV as a major public health threat by 2030 [4]. Rapid scale-up of DAA treatment for target populations with high HCV prevalence, such as prisoners and persons who use drugs (PWUD), is crucial for achieving elimination [5,6,7]. Injection drug users, who are at high risk for transmitting HCV, account for 70% of all new HCV cases [8], and modeling studies have shown that even modest increases in treatment of PWUD can reduce HCV prevalence and incidence [9, 10]. In Australia, high uptake of DAAs among PWUD has reduced the prevalence of active HCV infection from 51% in 2015 to 18% in 2019 [5]. Expanding treatment to other historically marginalized patients with HCV, such as patients with psychiatric disorders or a history of alcohol abuse, as well as those who are unemployed or have low to no education is also important for removing barriers to achieve elimination [11, 12].
The WHO recommends treating all adults with chronic hepatitis C (CHC) with a pangenotypic DAA [4], the advantages of which include simpler treatment regimens and broader access in comparison to nonpangenotypic regimens [2]. Similarly, current guidelines from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) and the European Association for the Study of the Liver (EASL) recommend broad treatment of nearly all individuals with CHC [13, 14]. Glecaprevir/pibrentasvir is an interferon-free, ribavirin-free, fixed-dose pangenotypic DAA combination approved in the USA and Europe in 2017 for the treatment of HCV genotypes (GT) 1–6, and currently available in 66 countries [15]. In clinical trials, glecaprevir/pibrentasvir demonstrated an overall sustained virologic response at post-treatment week 12 (SVR12) rate of 97.5% with a favorable safety profile [15,16,17]. Recently, the EXPEDITION-8 trial demonstrated that 8 weeks of glecaprevir/pibrentasvir is highly effective and well-tolerated in treatment-naïve patients with compensated cirrhosis (CC) [18], leading to the approval of 8-week glecaprevir/pibrentasvir for treatment-naïve patients with HCV and GT1–6 and CC [15]. Real‐world data on glecaprevir/pibrentasvir are limited, particularly in high-risk populations, including PWUD [19, 20].
In clinical practice, patient populations may be more diverse and potentially less adherent to treatment versus those in clinical trials [21], which could impact the safety and effectiveness of DAAs in these settings. Compared with the general population, people with HCV have a higher prevalence of comorbidity and multimorbidity [22], and patients with dual disorders, such as substance and/or alcohol abuse combined with psychiatric disorders, were historically excluded from registrational trials. It is therefore crucial to assess the effectiveness and safety of pangenotypic DAAs in real-world populations, particularly among PWUD and patients with other single or dual disorders. Real-world studies on glecaprevir/pibrentasvir conducted in single-country cohorts demonstrated high rates of SVR12 [19, 20, 23,24,25,26]; however, there may be a greater diversity of patient subpopulations and treatment patterns across multiple countries. Real-world data on the impact of treatment with DAAs on patient health-related quality of life (HRQOL) are also limited, although their impact on HRQOL in patients with CHC was documented in registration studies [27]. Patients with CHC were shown to have diminished HRQOL [28, 29], primarily related to the presence of fatigue and other common extrahepatic manifestations [30, 31]. Among PWUD, treatment of HCV with DAAs may improve patient-reported outcomes, including HRQOL [32], although limited data have been reported to date. Real-world clinical practice data on safety, effectiveness, and HRQOL in these patients is important to overcome barriers to treatment, to guide clinical decisions, and to support the 2030 WHO target of HCV elimination.
In this pooled analysis of ongoing, multinational, post-marketing observational studies (PMOS), we evaluated the real-world effectiveness, safety, and HRQOL of glecaprevir/pibrentasvir in adult patients with CHC from underserved populations, with an emphasis on PWUD and those patients with psychiatric disorders.
Methods
Study Design
This was an interim analysis of data pooled from AbbVie-sponsored PMOS across nine countries: Austria, Belgium, France, Greece, Israel, Italy, Poland, Portugal, and Switzerland (13 November 2017–31 January 2020), which began shortly after approval of glecaprevir/pibrentasvir. Eligible patients were followed from treatment initiation until 12 weeks after the end of treatment (EOT) or until premature discontinuation. Each study was conducted in accordance with local laws and regulations and received the required approvals from the responsible regulatory authorities, ethics committees, and/or competent authorities. Written informed consent was obtained from all included patients, including permission to publish personal health information while ensuring the identity of the individual remains confidential. The study protocol conformed to the ethical guidelines of the 1964 Declaration of Helsinki, and its later amendments. All authors had access to relevant data, and participated in the writing, review, and approval of the final manuscript.
Study Population
The PMOS included patients aged at least 18 years with HCV GT1–6 infection who received glecaprevir/pibrentasvir at the treating physician’s discretion according to local label, national or international recommendations, and/or local clinical practice; patients who were CHC treatment-naïve or had been previously treated with interferon, ribavirin, sofosbuvir, or DAA; and those without cirrhosis or with CC. The presence of cirrhosis was defined as METAVIR stage 4, or Knodell stage 4, or International Association for the Study of the Liver (IASL) stage 4, or Scheuer stage 4; or Ishak stage ≥ 5; or by transient elastography: FibroScan® (Echosens, Waltham, MA) ≥ 12.5 kPa; FibroTest (BioPredictive, Paris, France) ≥ 0.75; or acoustic radiation force impulse (ARFI) imaging ≥ 2.31.
Outcomes
Effectiveness
Effectiveness was measured as the percentage of patients achieving SVR12, defined as HCV ribonucleic acid (RNA) below the lower limit of quantification (LLOQ, < 50 IU/mL) at 12 ± 2 weeks (i.e., at least 70 days) after the last dose of glecaprevir/pibrentasvir. On-treatment virologic failure was defined as viral breakthrough or failure to suppress HCV replication. Viral breakthrough was defined as confirmed HCV RNA ≥ 100 IU/mL after HCV RNA < LLOQ during the treatment period or confirmed increase from nadir in HCV RNA (two consecutive HCV RNA measurements > 1 log10 IU/mL above nadir) at any time point during the treatment period. Failure to suppress was defined as patients with HCV RNA ≥ LLOQ at EOT with at least 6 weeks of treatment, where the HCV RNA value must be collected on or after 36 days of treatment (excludes patients counted in the breakthrough summary). Relapse was defined as confirmed HCV RNA ≥ LLOQ between EOT and 12 weeks after last actual dose of study drug (up to and including the SVR12 assessment time point) for a patient with HCV RNA < LLOQ at the final treatment visit who completed treatment (defined as study drug duration at least 52 days for 8 weeks, at least 77 days for 12 weeks, or at least 103 days for 16 weeks), excluding reinfection. Virologic failure was defined as “other” virologic failure if on-treatment HCV RNA measurements were missing and therefore it was not possible to determine if the failure was relapse or on-treatment failure.
Health-Related Quality of Life
Patient reported-outcomes (PROs) included the 36-Item Short-Form Health Survey (SF-36, consisting of eight domains and two aggregate component scores (physical component summary [PCS] and mental component summary [MCS]) (range 0–100; higher scores indicating better HRQOL) and the Fatigue Severity Scale (FSS) (scored 1–7) [33, 34]. FSS includes nine items relating to the extent of fatigue symptoms and their impact on patient functioning [33]. The total score is the mean of the nine item scores (range 1–7) with higher scores indicating greater fatigue. The percentage of patients achieving a minimally important difference (MID) in PROs from baseline to the SVR12 visit was reported [33, 35, 36]. MIDs were defined as an increase of at least 2.5 in PCS or MCS scores [36] and a decrease of at least 0.7 in FSS scores; thresholds considered clinically relevant [33].
Safety
Safety was assessed by monitoring adverse events (AEs) (including fatalities, and AEs causing study drug discontinuation) and abnormal laboratory measurements reported in the safety population. AEs were coded according to MedDRA 21.0 (Medical Dictionary for Regulatory Activities, McLean, VA) system organ class and preferred terms. An AE was considered treatment-emergent if the reported event began or worsened after initiation of glecaprevir/pibrentasvir through 30 days after glecaprevir/pibrentasvir dosing or if the date of onset was missing.
Statistical Analysis
The safety population included all patients meeting the inclusion criteria receiving at least one dose of glecaprevir/pibrentasvir, including those who were treated off label. The core population included patients from the safety population treated according to the current label at the time of study in accordance with their disease characteristics and with a known drug start date, excluding patients who were DAA-experienced. The core population with sufficient follow-up (CPSFU) included patients from the core population, excluding those who did not have an HCV RNA evaluation after post-treatment day 70 for reasons not related to effectiveness or safety (lost to follow-up or unavailable HCV RNA data). Patients included in the CPSFU had one of the following: HCV RNA data after post-treatment day 70 (not included if the drug end date was unknown), virologic failure (on-treatment virologic failure or post-treatment relapse), discontinued the study because of an AE, and had HCV RNA < 50 IU/mL at the last measurement.
Patient characteristics at baseline, including comorbidities and patient-reported co-medication use (Anatomical Therapeutic Chemical code 3), were summarized in the safety population. Summary statistics (n, mean, median, standard deviation [SD], minimum, maximum) were generated for continuous variables, and number and percentage of patients were reported for categorical variables. Patient-reported treatment adherence was calculated as the percentage of tablets taken relative to the total tablets expected to be taken. The percentage of patients achieving SVR12 was calculated for the CPSFU and a two-sided 95% confidence interval (CI) of the percentage was computed on the basis of the Wilson’s score method. Mean change in total score from baseline to the SVR12 visit and the number and percentage of patients with improvements greater than or equal to the MID from baseline to any visit through to the SVR12 visit, along with two-sided 95% CIs, were reported for all PROs in the core population. Effectiveness and PROs were reported in one or more of the following disorders, dual disorders, or triple disorder of interest: cirrhotic/non-cirrhotic, 8- or 12-week glecaprevir/pibrentasvir duration, illegal drug use (PWUD), psychiatric disorder, alcohol use, unemployment or low to no education; PWUD and psychiatric disorders; PWUD and alcohol use; and PWUD + unemployed + low to no education. In this analysis, key subgroups were defined as the following: PWUD as any patient-reported illicit drug use; alcohol use as consumption of more than two alcoholic drinks/day; and psychiatric disorder as a history of depression or bipolar disease, depression and suicide/self-jury, and anxiety. For treatment-naïve GT1–6 patients with cirrhosis, these analyses regard 12 weeks of glecaprevir/pibrentasvir as the label-recommended regimen because the study statistical analysis plan predated the approval of 8 weeks of glecaprevir/pibrentasvir for these patients.
All statistical analyses were conducted using the SAS® software package (version 9.4; SAS Institute Inc., Cary, NC, USA).
Results
Study Population
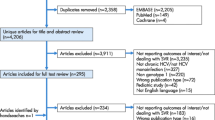
As of January 31, 2020, 2116 patients were enrolled: 2036 in the safety population, including 119 who were treated off label and excluded from the core population; 1917 in the core population; 1684 in the CPSFU (Fig. 1). Twelve percent of patients (n = 233) were excluded from the CPSFU, including 126 patients reported as lost to follow-up (Fig. 1). Ninety patients in the safety population with sufficient follow-up but treated off label were excluded from the CPSFU (Supplementary Table 1).
Patient selection. 190 patients treated off label had sufficient follow-up; SVR12 rate among these patients was 94.4% (85/90, 5 virologic failures). 2To date, 9 of these 13 patients have achieved SVR12 with no virologic failures. 3Patients with premature discontinuation due to AE and last HCV RNA < 50 IU/mL were included in the CPSFU. AE adverse event, CPSFU core population with sufficient follow-up, DAA direct-acting antiviral, HCV hepatitis C virus, RNA ribonucleic acid, SVR12 sustained virologic response at post-treatment week 12
Demographics and Clinical Characteristics
In the safety population, 1782 (87.5%) were non-cirrhotic and 1760 (86.7%) were HCV treatment-naïve (Table 1). As a result, 1701 (83.5%) patients were assigned to glecaprevir/pibrentasvir for 8 weeks, whereas 295 (14.5%) and 40 (2.0%) were assigned to glecaprevir/pibrentasvir for 12 and 16 weeks, respectively. In 2032 patients with available data, mean adherence to treatment was 99.7% ± 2.6%.
Comorbidities
At baseline, 11.0% of patients (n/N = 219/1990) were on stable opioid substitution therapy. Approximately one-third of the population (n/N = 697/2008; 34.7%) reported recreational drug use, which was inclusive of injection drug use. Among these, 168 had recent (within 12 months prior to screening) and 519 had former (more than 12 months prior to screening) recreational drug use. Overall (n = 2036), the five most common recreational drugs were heroin (n = 451; 22.5%), cocaine (n = 158; 7.9%), marijuana (n = 77; 3.8%), hashish (n = 58; 2.9%), and opioids (n = 27; 1.3%). Of 2036 patients who received at least one dose of glecaprevir/pibrentasvir, 200 (9.8%) had psychiatric disorders, 322 (18.5%) had a history of alcohol use, and 835 (45.0%) were unemployed or had low to no education (Table 2). Overall, 127 (6.2%) were PWUD with a psychiatric disorder, 223 (11.0%) were PWUD with a history of alcohol use, and 143 (7.0%) were PWUD who were unemployed and had low or no education (Table 2). A minority of patients were coinfected with human immunodeficiency virus (HIV; n/N = 111/2036; 5.5%) or hepatitis B virus (HBV; n/N = 25/2036; 1.2%).
Co-medications
Overall, 44.7% (n/N = 911/2036) of the overall population received at least one co-medication. Among 2036 patients, 312 (15.3%) received 1 co-medication, 431 (21.2%) received 2–4 co-medications, 131 (6.4%) received 5–8 co-medications, and 37 (1.8%) received more than 8 co-medications (Table 3). Additionally, approximately half of the PWUD population (n/N = 352/697; 50.5%) received at least one co-medication (Table 3). In PWUD, 121 (17.4%) received 1 co-medication, 174 (25.0%) received 2–4 co-medications, 47 (6.7%) received 5–8 co-medications, and 10 (1.4%) received more than 8 medications.
Effectiveness
In the CPSFU, the SVR12 rate was 98.0% (n/N = 1651/1684; 95% CI 97.3–98.6) overall, 98.1% (n/N = 1432/1459; 95% CI 97.3–98.7) in patients treated for 8 weeks, 97.0% (519/535; 95% CI 95.2–98.2) in PWUD, and 99.4% (159/160; 95% CI 96.5–99.9) in patients with psychiatric disorders (Fig. 2a). By GT, the SVR12 rate was 99.4% (890/895) for GT1, 99.4% (163/164) for GT2, 96.2% (429/446) for GT 3, and 99.4% (153/154) for GT4–6. Thirty-three patients did not achieve SVR12 (6 on-treatment virologic failures; 15 relapses; 6 other virologic failures; 2 with missing SVR12 data; and 4 discontinuations) (Supplementary Table 2). Of the 33 non-responders, 16 were PWUD, 1 patient had a history of psychiatric disorder, 11 had history of alcohol use, and 6 were PWUD and alcohol users. Five patients treated off label also had virologic failure (Supplementary Table 3). SVR12 rates were similarly high (> 95%) across subgroups of interest (Fig. 2a), number of co-medications within PWUD (Fig. 2b), and by class of co-medication (Fig. 2c).
SVR12 rates with glecaprevir/pibrentasvir treatment. a Overall and by subgroups of interest, b by number of co-medications for PWUD within the CPSFU, c by drug class of interest (PWUD in CPSFU). Error bars represent 95% confidence intervals. SVR12 rate was 29/33 in patients treated with G/P for 16 weeks. 1Patients who consumed more than two drinks/day. ARV antiretroviral, CC compensated cirrhosis, CPSFU core population with sufficient follow-up, G/P glecaprevir/pibrentasvir, PI protease inhibitor, OST opioid substitution treatment, PWUD persons who use drugs, SVR12 sustained virologic response at post-treatment week 12, TN treatment-naïve, VF virologic failure, wk week
Health-Related Quality of Life
In the core population, the mean ± SD change from baseline to the SVR12 visit was 2.4 ± 8.3 and 3.7 ± 10.8, for the SF-36 PCS and SF-36 MCS scores, respectively. In PWUD, the mean ± SD change from baseline to the SVR12 visit was 2.2 ± 8.6 and 3.9 ± 11.0 for SF-36 PCS and MCS scores, respectively. In patients with psychiatric disorder, the mean ± SD change from baseline to the SVR12 visit was 2.9 ± 8.2 and 5.8 ± 11.1 for SF-36 PCS and MCS scores, respectively. The percentages of patients who achieved improvements greater than or equal to the MID in PROs are presented in Fig. 3a–c. Fifty-three percent (52.6, n/N = 180/342) of PWUD achieved improvements greater than or equal to the MID in SF-36 MCS from baseline at the SVR12 visit. At the SVR12 visit, 64.0% (n/N = 57/89) and 68.0% (n/N = 34/50) of patients with psychiatric disorder or PWUD with psychiatric disorder, respectively, achieved improvements in SF-36 MCS scores from baseline.
Patients who achieved MID from baseline through SVR12 visits on SF-36: a MCS, b PCS, and c FSS. Error bars represent 95% confidence intervals. 1At least a 2.5 increase in PCS or MCS scores was considered to be a clinically relevant improvement. 2Patients who consumed more than two drinks/day. BL baseline, FSS fatigue severity scale, MCS mental component summary, MID minimally important difference, PCS physical component summary, PTW12 post-treatment week 12, SF-36 36-Item Short-Form Health Survey, SVR12 sustained virologic response at post-treatment week 12, PWUD persons who use drugs, wk week
Concomitant Medicines
In the core population, more than 96.5% of PWUD achieved SVR12 regardless of the number of concomitant medications. Patients without any concomitant medications achieved an SVR12 rate of 96.6% (253/262), and in patients with 1, 2–4, 5–8, and more than 8 concomitant medications SVR12 rates were 98.0% (97/99), 96.9% (125/129), 97.3% (36/37), and 100% (8/8), respectively.
Safety
Glecaprevir/pibrentasvir had a favorable safety profile, with one serious AE (SAE; acute pericarditis beginning 1 month after study drug initiation, patient completed study drug) considered treatment-related by the investigator. The patient with acute pericarditis had no underlying risk factor, medical history, or family history relevant to this SAE. The patient was hospitalized and treated with Aspegic and colchicine, and was listed as making a complete recovery. A low rate of AEs leading to premature study drug discontinuation (n = 10; 0.5%) was observed (Table 4). DAA-related AEs leading to discontinuation of study drug (n = 6 overall, 0.3%) were as follows: nausea (n = 1); dizziness, nausea, and malaise (n = 1); loss of appetite, fatigue, and muscle cramps (n = 1); increased creatinine value (n = 1); ascites (n = 1); headache, nausea, and vomiting (n = 1). One hepatic decompensation event (ascites) leading to discontinuation of study drug developed in one patient with baseline Child–Pugh A, baseline evidence of decreased synthetic function (hypoalbuminemia), and risk factors for passive congestion of the liver (idiopathic pulmonary fibrosis). The patient with increased creatinine values (2.91 mg/dL at 54 days prior to first dose, 3.29 mg/dL at 28 days after first dose, 3.42 mg/dL at 83 days after first dose, and 3.45 mg/dL at 152 days after first dose) discontinued treatment 70 days after treatment initiation, had a renal transplant in 1994, and several comorbidities including Alport syndrome, ischemic heart disease, and osteoporosis. Eighteen SAEs occurred, 17 of which were not considered treatment-related (Supplementary Table 4); one SAE unrelated to the study drug was observed in the 90 patients in the off-label population with sufficient follow-up (heart failure exacerbation due to respiratory tract infection). The most common AEs were asthenia (n = 43; 2.1%), fatigue (n = 41; 2.0%), headache (n = 40; 2.0%), and nausea (n = 20; 1.0%). Five deaths were reported, none of which were related to treatment with glecaprevir/pibrentasvir.
Elevations of alanine aminotransferase were uncommon and did not occur with total bilirubin elevations; no cases consistent with drug-induced liver injury were identified. Elevations of total bilirubin were rare and consistent with the labeled glecaprevir/pibrentasvir-mediated inhibition of bilirubin transport and metabolism. Bilirubin elevations did not occur with events of hepatic decompensation. Of the patients with elevated alanine aminotransferase, elevations returned to baseline after completion of treatment with glecaprevir/pibrentasvir in one patient and remained elevated after treatment completion in a second patient.
Discussion
Real-world studies provide valuable information regarding treatment effectiveness and safety in routine clinical practice, where patient populations are more heterogeneous and more inclusive of marginalized populations, especially PWUD and patients with dual disorders, than those enrolled in clinical trials. This pooled analysis of patient-level data from ongoing PMOS studies demonstrates that glecaprevir/pibrentasvir is highly effective with a favorable safety profile across patient populations, including in marginalized patients in routine clinical practice from nine countries. The overall SVR12 rate was 98.0%, which is consistent with that seen in the glecaprevir/pibrentasvir registration trials (97.5%), and more recently in the EXPEDITION-8 trial (97.7% in the intention-to-treat population) [15, 18]. This rate remained high (> 95%) irrespective of treatment duration, cirrhosis, or subgroups of interest, including patients with dual disorders. Thirty-three patients did not achieve SVR12, including 6 on-treatment virologic failures (< 1%), 15 relapses (1.2%), and 6 other virologic failures (< 1%), of which most were treatment-naïve and non-cirrhotic and 16 were PWUD. Our results demonstrate that glecaprevir/pibrentasvir is an effective treatment for PWUD with CHC across a broad range of prescribed or non-prescribed drugs, as well as in other underserved patient populations.
Although real-world studies of glecaprevir/pibrentasvir use in routine clinical practice are currently limited, our results are consistent with findings from recently published or presented real-world studies of glecaprevir/pibrentasvir in Germany [19, 37], Italy [20, 25], USA [23, 26], and Scotland [24]. Similarities include the high percentages of treatment-naïve non-cirrhotic patients (> 77%), a majority of patients with HCV GT1, and median age greater than 45 years in this PMOS and in the other cohorts. The small proportion of patients with CC in all cohorts, except the 2019 German and the US studies focusing specifically on patients with cirrhosis, is reflective of the current disease burden of newly diagnosed HCV-infected patients as many patients with CC may have been treated previously. Our study demonstrated a high SVR12 rate among all PWUD, capturing all drug users beyond those who inject heroin, which is a key population for HCV elimination. One-third (35%, n/N = 697/2008) of the patients in our study were PWUD. Several of the previous studies did not specifically evaluate SVR12 rate among PWUD or had only small percentages of patients reporting active drug use [19, 20, 23, 25, 26, 37]. It may be that active drug use was underreported in those studies or that active drug use remains a barrier to DAA treatment in real-world clinical settings across many countries.
In terms of treatment, the WHO HCV elimination 2030 target is achievable because of the availability of highly effective and well-tolerated pangenotypic DAAs [38]. However, only a small number of countries are on target to achieve this goal, often because of a lack of diagnosis and treatment [38]. Large decreases in HCV prevalence and incidence are possible with increased screening and successful treatment of CHC, particularly among groups at high risk for transmission [39]. Within the community, there are groups of patients that are more difficult to engage with healthcare, as they are often underserved patients who are typically excluded from clinical trials because of their lifestyles and comorbidities [11]. This group includes PWUD, patients with psychiatric disorders, unemployed patients, those with a history of incarceration, those with low to no education, and alcohol users [11]. It is essential to cover these patients with medical care in order to achieve HCV elimination [40]. PWUD bear a high burden of chronic HCV infection, with injection drug use accounting for 70% of all new infections which may be attributed to the opioid epidemic [5, 8]. In comparison to the general public, patients with HCV have a higher burden of comorbidities and multimorbidity, including a much higher burden of mental health and substance and/or alcohol abuse disorders [22]. Modeling studies have shown that treatment and successful cure of persons who inject drugs, a population at high risk for transmission of HCV, may decrease prevalence and incidence rates of HCV infections [9, 41, 42]. Historically there have been barriers to treating these patients including stigma, patient’s beliefs and lack of information regarding treatment, and perceived lack of adherence and efficacy in these patients [6, 43, 44]. In these PMOS, glecaprevir/pibrentasvir treatment was well tolerated and highly effective with an SVR12 rate of greater than 95% in PWUD as well as PWUD dual disorders including PWUD in combination with psychiatric disorders and PWUD and have low to no education or are unemployed. Among PWUD, the SVR12 rate was consistently high (> 96%) regardless of the number of co-medications.
Similarly, in other marginalized groups, glecaprevir/pibrentasvir was highly effective, as evidenced by the high rates of SVR12 (> 95%) in patients with alcohol use, low to no education, or who were unemployed. The SVR12 rate (99%) was also high among patients with psychiatric disorders in our study. Treatment and management of HCV in these groups, including implementation of harm reduction strategies [45], will be critical for preventing future infections and achieving the goal of HCV elimination. The data from this real-world analysis reinforces WHO, AASLD-IDSA, and EASL guideline suggestions that these patients should be treated [13, 14, 46], and demonstrates that glecaprevir/pibrentasvir may be an effective and safe treatment option for PWUD and PWUD dual disorders as well as other marginalized patient populations in real-world clinical practice settings. Microelimination projects for HCV in a variety of countries may be successful in reducing the burden of HCV, further supporting the importance of targeting these marginalized populations to reduce HCV prevalence and achieve HCV elimination [47,48,49,50]. Interestingly, there were also 192 (10%) patients with rare genotypes (GT4–6). The SVR12 rate was 99.4% among these patients. This result was similar to those reported in a previous clinical trial where glecaprevir/pibrentasvir achieved high SVR12 rates (97.6%) in patients with GT4–6 [51]. The prevalence of HCV GT4–6 is increasing in North America and Europe as a result of migration from the Middle East, Africa, and Southeast Asia [52]. With the successful development of pangenotypic DAAs, there is a unique opportunity to achieve global HCV elimination among patients with rare genotypes as well as other marginalized populations.
Most patients in this study and other reported registries were non-cirrhotic and treatment-naive and received glecaprevir/pibrentasvir for 8 weeks. In this analysis, 408 patients in the PWUD population were treatment-naïve and received treatment for 8 weeks. The SVR12 was high (> 95%) among patients treated for 8 weeks. Thirteen treatment-naïve patients with CC received 8-week glecaprevir/pibrentasvir, of which nine achieved SVR12 with no virologic failures (SVR12 for the remaining patients are pending). Previous real-world data have also demonstrated high SVR12 rates among patients receiving 8-week glecaprevir/pibrentasvir [19, 23,24,25,26, 37, 43]. These data suggest that glecaprevir/pibrentasvir may be an appropriate and effective treatment option for all patients with CHC, including PWUD and other underserved populations. Indeed, glecaprevir/pibrentasvir is now available in a shorter, 8-week once-daily option for treatment-naïve patients with CC and HCV GT1–6 [15]. This is supported by data from the EXPEDITION-8 study with an SVR12 rate of 97.7% in GT1–6, and one virologic failure [18]. There are many potential benefits to shorter duration treatments for HCV, including the ability to reach patients in a broader range of community settings, improve adherence rates [53], and reduce medical costs. In particular, the healthcare resources saved with a shortened treatment duration, in terms of reduced number of visits with specialists, costs, and related complications (extrahepatic and hepatic), have the potential to increase the number of patients treated within specific healthcare budgets [54]. Increasing the number of patients who are treated for HCV may reduce the time until HCV elimination is achieved [54]. There are other settings not included in this study in which 8-week treatment may prove to be beneficial including in prisons, particularly where patients are transferred or released during treatment, and in refugee camps where the prevalence of HCV is reported to be relatively high and patient engagement difficult [55, 56].
In addition to the high rates of SVR, improvements in HRQOL were also observed in patients with CHC treated with glecaprevir/pibrentasvir from baseline to the SVR12 visit, with the proportion of patients meeting the MID threshold higher at SVR12 than EOT. The mean change from baseline to the SVR12 visit in SF-36 MCS was clinically meaningful. Greater than 45% of patients experienced a clinically meaningful improvement in the mental components of HRQOL; this was most evident in patients with psychiatric disorders, PWUD combined with psychiatric disorders, and PWUD combined with alcohol users. Previously, treatment of HCV with DAAs was demonstrated to improve PROs, including HRQOL, particularly among PWUD and patients on opioid substitution therapy [32]. A similar percentage (40.8%) of patients experienced a clinically meaningful decrease in fatigue. The impact of fatigue on patients with CHC was previously documented in clinical trials [27]. These results suggest that, in the short term, successful treatment with glecaprevir/pibrentasvir alleviates some symptoms associated with CHC infection. This evidence may support the treatment of patients with extrahepatic manifestations for whom HRQOL is reduced [57].
Although AEs may be underreported in real-world clinical settings, glecaprevir/pibrentasvir’s safety profile in this study is consistent with that observed in the registration trials [15, 19, 20]. Overall, glecaprevir/pibrentasvir had a favorable safety profile: there was a low rate of SAEs reported with only one SAE (< 0.1%) considered treatment-related observed as well as a low rate of study drug discontinuation as a result of AEs. Observed laboratory abnormalities and hepatic decompensation events were also rare. Bilirubin elevations were consistent with that described in the label from the glecaprevir/pibrentasvir inhibition of bilirubin transport and metabolism. No cases consistent with drug-induced liver injury were identified and the single reported case of hepatic decompensation (ascites) in a patient with CC may be attributable to the natural course of the patient’s underlying liver disease.
Limitations
Similar to other real-world observational studies, bias may exist in the reporting and collection of patient-level information. Adverse events, non-adherence, and recreational drug use are often underreported in real-world settings. Recreational drug use and adherence to treatment were patient-reported. This study had a high level of patients who were lost to follow-up, which may impact the rates of SVR12 observed and may be of concern in populations with ongoing risk who have potential for reinfection.
Conclusion
Our results demonstrate that glecaprevir/pibrentasvir is a well-tolerated and highly effective pangenotypic treatment option for a broad range of marginalized patients with CHC and single or dual disorders. These real-world results complement those seen in the glecaprevir/pibrentasvir registrational program and close current gaps in clinical knowledge regarding impact on HRQOL. Our results further support the use of 8-week short-course pangenotypic glecaprevir/pibrentasvir in underserved patients including those with substance abuse and psychiatric comorbidities, addressing barriers to HCV elimination including widespread stigma and lack of confidence in treating underserved patient groups and may provide the impetus to meet the WHO elimination targets.
Change history
16 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40121-021-00529-0
References
Asselah T, Marcellin P, Schinazi RF. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int. 2018;38(suppl 1):7–13.
Li G, De Clercq E. Current therapy for chronic hepatitis C: the role of direct-acting antivirals. Antiviral Res. 2017;142:83–122.
WHO. Global hepatitis report, 2017. World Health Organization. 2017. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf. Accessed 10 Feb 2021.
WHO. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection, July 2018. 2018. https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2018/en/. Accessed 10 Feb 2021.
Dore GJ, Trooskin S. People with hepatitis C who inject drugs—underserved, not undeserving. N Engl J Med. 2020;383(7):608–11.
Lazarus JV, Safreed-Harmon K, Thursz MR, et al. The micro-elimination approach to eliminating hepatitis C: strategic and operational considerations. Semin Liver Dis. 2018;38(3):181–92.
Pol S, Parlati L. Treatment of hepatitis C: the use of the new pangenotypic direct-acting antivirals in “special populations.” Liver Int. 2018;38(suppl 1):28–33.
AASLD-IDSA. Key populations: identification and management of HCV in people who inject drugs (updated November 6, 2019). https://www.hcvguidelines.org/unique-populations/pwid. Accessed 10 Feb 2021.
Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–609.
Zelenev A, Mazhnaya A, Basu S, Altice FL. Hepatitis C virus treatment as prevention in an extended network of people who inject drugs in the USA: a modelling study. Lancet Infect Dis. 2018;18(2):215–24.
Edmunds BL, Miller ER, Tsourtos G. The distribution and socioeconomic burden of Hepatitis C virus in South Australia: a cross-sectional study 2010–2016. BMC Public Health. 2019;19(1):527.
Roose RJ, Cockerham-Colas L, Soloway I, Batchelder A, Litwin AH. Reducing barriers to hepatitis C treatment among drug users: an integrated hepatitis C peer education and support program. J Health Care Poor Underserved. 2014;25(2):652–62.
AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed 10 Feb 2021.
EASL. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461–511.
Maviret EU. Maviret. 2020. https://www.ema.europa.eu/en/documents/product-information/maviret-epar-product-information_en.pdf. Accessed 20 Dec 2020.
Asselah T, Kowdley KV, Zadeikis N, et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol. 2018;16(3):417–26.
Zeuzem S, Foster GR, Wang S, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 2018;378(4):354–69.
Brown RS Jr, Buti M, Rodrigues L, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naive patients with chronic HCV genotypes 1–6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol. 2020;72(3):441–9.
Berg T, Naumann U, Stoehr A, et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C infection: data from the German Hepatitis C-Registry. Aliment Pharmacol Ther. 2019;49(8):1052–9.
D’Ambrosio R, Pasulo L, Puoti M, et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir in 723 patients with chronic hepatitis C. J Hepatol. 2019;70(3):379–87.
Zoulim F, Liang TJ, Gerbes AL, et al. Hepatitis C virus treatment in the real world: optimising treatment and access to therapies. Gut. 2015;64(11):1824–33.
Cooper CL, Galanakis C, Donelle J, et al. HCV-infected individuals have higher prevalence of comorbidity and multimorbidity: a retrospective cohort study. BMC Infect Dis. 2019;19(1):712.
Belperio P, Shahoumian T, Loomis T, Mole L, Backus L. Real-world effectiveness of glecaprevir/pibrentasvir in 1941 patients with hepatitis C genotypes 1 through 4. Hepatology. 2018;68(suppl 1):A417–A418 (abstract 703).
Marra F, Boyle A, McDonald N, et al. 8 weeks of glecaprevir/pibrentasvir is effective and well tolerated in non-GT3 HCV patients with cirrhosis. Hepatology. 2019;70(suppl 1):A957–A958 (abstract 1588).
Persico M, Aglitti A, Milella M, et al. Real-life glecaprevir/pibrentasvir in a large cohort of patients with hepatitis C virus infection: the MISTRAL study. Liver Int. 2019;39(10):1852–9.
Steve F, Jens K, Steven M, et al. Effectiveness of 8-week glecaprevir/pibrentasvir for treatment naive, compensated cirrhotic patients with chronic hepatitis C infection. Adv Ther. 2020;37:2267–74.
Cacoub P, Bondin MI, Hayes O, Pinsky B, Negro F. Quality of life in patients with psychiatric disorders: pooled analysis from glecaprevir/pibrentasvir registrational studies. Heptology. 2018;68(suppl 1):A94–A95 (abstract 150).
Gutteling JJ, de Man RA, van der Plas SM, Schalm SW, Busschbach JJ, Darlington AS. Determinants of quality of life in chronic liver patients. Aliment Pharmacol Ther. 2006;23(11):1629–35.
Loria A, Doyle K, Weinstein AA, et al. Multiple factors predict physical performance in people with chronic liver disease. Am J Phys Med Rehabil. 2014;93(6):470–6.
Gutteling JJ, Duivenvoorden HJ, Busschbach JJ, de Man RA, Darlington AS. Psychological determinants of health-related quality of life in patients with chronic liver disease. Psychosomatics. 2010;51(2):157–65.
Younossi ZM, Stepanova M, Henry L, Nader F, Hunt S. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111(6):808–16.
Stepanova M, Thompson A, Doyle J, Younossi I, de Avila L, Younossi ZM. Hepatitis C virus–infected patients receiving opioid substitution therapy experience improvement in patient-reported outcomes following treatment with interferon-free regimens. J Infect Dis. 2017;217(7):1033–43.
Rosa K, Fu M, Gilles L, et al. Validation of the Fatigue Severity Scale in chronic hepatitis C. Health Qual Life Outcomes. 2014;12:90.
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83.
Bjorner JB, Wallenstein GV, Martin MC, et al. Interpreting score differences in the SF-36 Vitality scale: using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin. 2007;23(4):731–9.
Strand V, Fiorentino D, Hu C, Day RM, Stevens RM, Papp KA. Improvements in patient-reported outcomes with apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of moderate to severe psoriasis: results from a phase IIb randomized, controlled study. Health Qual Life Outcomes. 2013;11:82.
Wedemeyer H, Erren P, Naumann U, et al. Glecaprevir/pibrentasvir is effective and well tolerated in hepatitis c patients with cirrhosis: real-world experience from the German Hepatitis C-Registry. Hepatology. 2019;70(suppl 1):A917–A918 (abstract 1525).
Razavi H, Sanchez Gonzalez Y, Yuen C, Cornberg M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int. 2020;40(3):522–9.
Wedemeyer H, Duberg AS, Buti M, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepatitis. 2014;21(suppl 1):60–89.
Roncero C, Ryan P, Littlewood R, et al. Practical steps to improve chronic hepatitis C treatment in people with opioid use disorder. Hepatic Med Evid Res. 2019;11:1–11.
Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57(suppl 2):S39–45.
Martin NK, Vickerman P, Dore GJ, Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Curr Opin HIV AIDS. 2015;10(5):374–80.
Lampertico P, Peck-Radosavljevic M, Bondin M, et al. Addressing barriers to hepatitis c virus (HCV) elimination: real-world outcomes in historically underserved patients with chronic HCV infection treated with glecaprevir/pibrentasvir. Hepatology. 2019;70(suppl 1):954A (abstract 1583).
Palma-Álvarez RF, Ros-Cucurull E, Grau-López L, et al. Difficulties in initiating hepatitis C treatment in patients with opioid usdisorder: patient’s perspective. Heroin Addict Relat Clin Probl. 2019;21:47–51.
Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402–11.
WHO. Progress report on access to hepatitis C treatment 2018. https://apps.who.int/iris/bitstream/handle/10665/260445/WHO-CDS-HIV-18.4-eng.pdf;jsessionid=AC7402ADBBC3BA2404E5DF4768BE7F9B?sequence=1. Accessed 10 Feb 2021.
Benzaken AS, Girade R, Catapan E, et al. Hepatitis C disease burden and strategies for elimination by 2030 in Brazil. A mathematical modeling approach. Braz J Infect Dis. 2019;23(3):182–90.
Calleja Panero JL, Lens Garcia S, Fernandez Bermejo M, Crespo J. Definition of the profiles of hepatitis C virus patients based on the identification of risky practices in Spain. Rev Esp Enferm Dig. 2019;111(10):731–7.
Chen Q, Ayer T, Bethea E, et al. Changes in hepatitis C burden and treatment trends in Europe during the era of direct-acting antivirals: a modelling study. BMJ Open. 2019;9(6):e026726.
Wade AJ, Doyle JS, Gane E, et al. Outcomes of treatment for hepatitis C in primary care, compared to hospital-based care: a randomized, controlled trial in people who inject drugs. Clin Infect Dis. 2020;70(9):1900–6.
Asselah T, Lee SS, Yao BB, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients with chronic hepatitis C virus genotype 5 or 6 infection (ENDURANCE-5,6): an open-label, multicentre, phase 3b trial. Lancet Gastroenterol Hepatol. 2019;4(1):45–51.
Asselah T, Hassanein T, Waked I, Mansouri A, Dusheiko G, Gane E. Eliminating hepatitis C within low-income countries—the need to cure genotypes 4, 5, 6. J Hepatol. 2018;68(4):814–26.
Akiyama MJ, Norton BL, Arnsten JH, Agyemang L, Heo M, Litwin AH. Intensive models of hepatitis C care for people who inject drugs receiving opioid agonist therapy: a randomized controlled trial. Ann Intern Med. 2019;170(9):594–603.
Feld JJ, Sanchez Gonzalez Y, Pires AG, Ethgen O. Clinical benefits, economic savings and faster time to HCV elimination with a simplified 8-week treatment and monitoring program in chronic F0–F3 naïve patients in the US. Hepatology. 2018;68(suppl 1):2 (abstract 688).
Aspinall EJ, Mitchell W, Schofield J, et al. A matched comparison study of hepatitis C treatment outcomes in the prison and community setting, and an analysis of the impact of prison release or transfer during therapy. J Viral Hepat. 2016;23(12):1009–16.
Galli M, Ridolfo A, van denBogaart L, Negri C, Giacomelli A. HCV and immigration in Italy. Acta Biomed. 2018;89(Suppl 10):19–32.
Negro F, Forton D, Craxì A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149(6):1345–60.
Acknowledgements
We thank the participants of these studies. Glecaprevir was identified by AbbVie and Enanta.
Funding
AbbVie sponsored the study; contributed to its design; and participated in the collection, analysis, and interpretation of the data and in the writing, reviewing, and approval of the publication. AbbVie funded the Rapid Service and Open Access of this publication. All authors had access to relevant data, and participated in the writing, review, and approval of the publication. No honoraria or payments were made for authorship.
Medical Writing and/or Editorial Assistance
Medical writing support was provided by Brandy Menges, PhD, and Tom Owen, PhD, of Fishawack Communications Ltd, part of Fishawack Health, funded by AbbVie.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Mark Bondin, John Marcinak conceived or designed the trial. Zhenzhen Zhang led statistical analyses. Alessio Aghemo, Yves Horsmans, Stefan Bourgeois, Michael Gschwantler, Harald Hofer, Nasser Semmo, Francesco Negro, Ella Veitsman, Rawi Hazzan, Konstantinos Mimidis, Ioannis Goulis, Nuno Marques, Robert Flisiak, Wlodzimierz Mazur, Carlos Roncero, Fiona Marra, Georges Philippe Pageaux, Tarek Asselah, and Pietro Lampertico recruited and treated patients in the trial. All authors reviewed and approved the final version of the article.
Prior Presentation
Data were presented in part at the American Association for the Study of Liver Diseases’ The Liver Meeting Digital Experience, November 13–16, 2020.
Disclosures
Alessio Aghemo: Grant support: AbbVie and Gilead; Advisory board/Speaker: AbbVie, Alfasigma, Gilead, Intercept, and MSD. Yves Horsmans: Consultant: AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck. Stefan Bourgeois: Advisory board member and speaker’s bureau: AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and MSD. Michael Gschwantler: Advisor/Speaker: AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and MSD; Research support: AbbVie, Gilead, and MSD. Harald Hofer: Advisory board/speaker fee: AbbVie, Gilead, and MSD. Nasser Semmo: Advisory board: Gilead and MSD; Speaker: AbbVie. Francesco Negro: Advisor: AbbVie, Gilead, and Merck; Research support: Gilead. Mark Bondin, Zhenzhen Zhang, and John Marcinak: Employees of AbbVie, Inc. and may hold stock or stock options. Ella Veitsman has nothing to disclose. Rawi Hazzan has nothing to disclose. Konstantinos Mimidis: Advisory boards/lectures: AbbVie. Ioannis Goulis has nothing to disclose. Nuno Marques: Advisory board: AbbVie, Gilead, Janssen, MSD, and ViiV Healthcare. Robert Flisiak: Advisory and speaker honoraria during the last year from: AbbVie, Gilead, Merck, and Roche. Wlodzimierz Mazur: Lecture fees: AbbVie, Gilead, and Merck; Research funding: AbbVie, Gilead, and Merck; Consultancy: AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Merck, and Roche. Carlos Roncero: Received fees to give lectures for Janssen-Cilag, Ferrer-Brainfarma, Indivior, Lundbeck, Otsuka, Servier, GSK, Astra, Gilead, MSD, Sanofi, Exceltis, AbbVie, Takeda, Rubio and Casein. He has received financial compensation for his participation as consultant or a board member of Lundbeck, Gilead, MSD, Mundipharma, Indivior, Exceltis, Martindale, Camurus, Gebro, and AbbVie. He has carried out the PROTEUS project, which was funded by a grant from Reckitt-Benckiser/Indivior and the COSTEDOPIA project, which was funded by Indivior. He received two medical education grants from Gilead. Fiona Marra: Received speaking and teaching fees: AbbVie, Gilead, MSD and ViiV Healthcare. Georges Philippe Pageaux: Speaker for AbbVie, Gilead, and Intercept. Tarik Asselah: Advisor/Speaker/Investigator: AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Merck, and Roche. Pietro Lampertico: Speaker bureau and/or advisory board: AbbVie, Alnylam, Arrowhead, Bristol-Myers Squibb, Eiger BioPharmaceuticals, Gilead Sciences, GSK, Janssen, MSD, MYR Pharmaceuticals, Roche, and Spring Bank.
Compliance with Ethics Guidelines
Each study was conducted in accordance with local laws and regulations and received the required approvals from the responsible regulatory authorities, ethics committees, and/or competent authorities. Written informed consent was obtained from all included patients, including permission to publish personal health information while ensuring the identity of the individual remains confidential. The study protocol conformed to the ethical guidelines of the 1964 Declaration of Helsinki, and its later amendments. All authors had access to relevant data, and participated in the writing, review, and approval of the final manuscript.These multinational post-marketing observational studies are registered with ClinicalTrials.gov, number NCT03303599.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Aghemo, A., Horsmans, Y., Bourgeois, S. et al. Real-World Outcomes in Historically Underserved Patients with Chronic Hepatitis C Infection Treated with Glecaprevir/Pibrentasvir. Infect Dis Ther 10, 2203–2222 (2021). https://doi.org/10.1007/s40121-021-00455-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00455-1