Abstract
Background
The goal of this study was to investigate the prevalence and factors associated with persistent viral shedding (PVS) in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Methods
This was a prospective observational study including all consecutive adults hospitalized with SARS-CoV-2 infection. When the first nasopharyngeal swab was positive for SARS-CoV-2 RNA (day 0), additional samples were obtained on days + 3, + 5, + 7 and then once every 7 days until virus detection was negative. PVS was defined as the duration of shedding of at least 21 days after diagnosis. The primary endpoint of this study was the prevalence of PVS.
Results
Data were obtained regarding 121 consecutive hospitalized patients with SARS-CoV-2 infection (median age 66 years, male sex 65.3%). Overall, the prevalence of PVS was 38% (46/121 patients). According to univariate analysis, factors associated with PVS were immunosuppression (6.7% vs 21.7%, p = 0.02), increased interleukin-6 (IL-6) levels (≥ 35 ng/ml) at the time of diagnosis (43.4% vs 67.3%, p = 0.02), time from onset of symptoms to diagnosis (median days 7.0 vs 3.5, p = 0.001), intensive care unit admission (22.7% vs 43.5%, p = 0.02), and need for invasive mechanical ventilation (20.0% vs 41.3%, p = 0.01). The multivariate analysis indicated that immunosuppression, increased IL-6 levels at the time of diagnosis, time from onset of symptoms to diagnosis, and need for mechanical ventilation were independent factors associated with PVS.
Conclusions
PVS was detected in up to 38% of hospitalized patients with SARS-CoV-2 infection and was strongly associated with immunosuppression, increased IL-6 levels, and the need for mechanical ventilation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
SARS-CoV-2 viral shedding usually occurs within 21 days but several patients experience persistent virus shedding (PVS). |
We found that 38% of hospitalized adult patients with SARS-CoV-2 infection had persistent viral shedding. |
Immunosuppression and increased IL-6 levels at the time of diagnosis slow viral clearance and are associated with longer hospital stay and poorer outcome. |
Future investigations analyzing the potential for transmission in patients with PVS are required. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13148453.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has recently emerged as a new cause of acute respiratory disease [1,2,3], whose clinical spectrum encompasses asymptomatic infection, mild upper respiratory tract illness, and severe pneumonia with respiratory failure and even death [4,5,6,7].
Viral clearance in the respiratory tract usually occurs within 21 days [8, 9]. However, a variable proportion of patients with SARS-CoV-2 infection may experience persistent virus shedding (PVS) lasting up to 60–80 days after diagnosis [10,11,12]. Unfortunately, our understanding of why some patients with SARS-CoV-2 infection do or do not present with PVS has not been fully investigated and PVS remains an entity of uncertain clinical significance [13,14,15,16,17,18,19]. Literature on this topic is scarce and difficult to interpret because of the heterogeneity of definitions proposed [15,16,17,18,19] or different specimens collected for RNA detection (upper versus lower respiratory tract samples) [15].
In this study, we analyzed viral shedding data of adult patients with SARS-CoV-2 infection admitted to our hospital. Our aims were to investigate (1) the prevalence of PVS and (2) the association between PVS and patients’ underlying diseases, interleukin-6 (IL-6) levels at the time of diagnosis, and clinical outcome. We hypothesized that immunosuppressed patients with marked cytokine activation would be more likely to present with persistent SARS-CoV-2 RNA shedding.
Methods
Patients and Study Design
This was a prospective observational study of patients with laboratory-confirmed SARS-CoV-2 infection who were consecutively admitted to San Martino Policlinico Hospital (Genoa, Italy) from February 25, 2020 through March 25, 2020. Our institution is a 1200-bed teaching hospital with a full range of clinical services and attending an urban population of approximately 400,000 persons. The study was carried out in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of Liguria Region (N. CER Liguria 114/2020—ID 10420). All patients provided verbal informed consent because of isolation precautions for performing nasopharyngeal swab according to the local protocol approved by hospital authorities, for data collection and for participation in the study.
Test Performed
As soon as the first nasopharyngeal swab was positive for SARS-CoV-2 RNA (day 0), designated research doctors obtained the patient’s consent and managed each case according to routine clinical care, until death or hospital discharge. Consecutive nasopharyngeal swabs for SARS-CoV-2 real-time reverse transcriptase (RT)-PCR were obtained at day + 3, day + 5, day + 7 ,and then once every 7 days until viral detection was negative. If a first nasopharyngeal swab was negative, a second one was repeated after 24 h in order to confirm its negativity and, potentially, discontinue droplet precautions in patients with clinical resolution of the symptoms. Additionally, research doctors requested routine coagulation, and biochemical blood tests at day 0 and when clinically indicated.
Exclusion Criteria
Exclusion criteria included (1) patients aged less than 18 years; (2) patients with positive nasopharyngeal swabs who were discharged early (less than 21 days from diagnosis) into fiduciary isolation (also known as self-isolation); (3) death within the first 21 days after diagnosis without evidence of SARS-CoV-2 RNA clearance; (4) missing virologic data at any study time point.
Standard of Care and Treatment Procedures
During the study period, patients with suspected SARS-CoV-2 infection were managed and treated according to a local protocol. Briefly, nasopharyngeal swabs were taken in all symptomatic patients with suspected SARS-CoV-2 infection. Patients were only admitted to the hospital if they presented with acute respiratory insufficiency (PaO2 < 60 mmHg at rest in ambient air) or if the exacerbation of their underlying disease or severe symptoms were considered unmanageable at home. According to local guidelines, short-term antibiotic coverage and hydroxychloroquine 400 mg bid p.o. were recommended to all patients. Darunavir/ritonavir 800/100 once daily for 10 days was also recommended until March 24. Thereafter, the combination darunavir/ritonavir was withdrawn on the basis of published data regarding possible lack of efficacy of protease inhibitors for treating SARS-CoV-2 infections [20]. Since March 11 a single intravenous infusion of tocilizumab (dose 8 mg/kg) was also considered in the case of severe COVID-19 pneumonia. Moreover, following an internal review of risks and benefits of steroid treatment in patients with severe COVID-19, since March 19 intravenous administration of methylprednisolone (1 mg/kg for 5 days, followed by 0.5 mg/kg for another 5 days) was included in the protocol and administered in the emergency department in patients with severe pneumonia due to SARS-CoV-2.
Data Collected and Definitions
Detailed information regarding data collected and definitions used in the present study are provided in the supplementary material. Briefly, clinical data were recorded with the use of a pre-established protocol that included the following: demographics; underlying disease; clinical, laboratory and radiological findings at presentation; data on treatment; SARS-CoV-2-related complications; and outcome.
The duration of shedding was calculated on the basis of the time elapsed between the first positive nasopharyngeal swab and the first of two consecutive negative swabs taken 24 h apart. Persistent viral shedding was a priori defined as the duration of shedding of at least 21 days, which was recently described to be the median shedding duration of SARS-CoV-2 in an unselected group of patients [8, 9, 14].
Immunosuppression was defined as the presence of either (1) HIV infection and/or (2) hematological malignancy and/or (3) chronic treatment with immunosuppressant drugs and/or (4) a solid organ transplantation.
Main Outcomes Measure
The primary endpoint was the PVS among our study population including hospitalized adult patients with SARS-CoV-2.
The secondary endpoint was to investigate the association between PVS and patients’ underlying diseases (hypertension, cardiovascular disease, immunosuppression, diabetes mellitus, chronic kidney disease, cerebrovascular disease, chronic obstructive lung disease, solid tumor), inflammatory biomarkers at the time of diagnosis (D-dimer, C-reactive protein (CRP), and IL-6 levels), and clinical outcome (need for non-invasive respiratory support or invasive mechanical ventilation, intensive care unit (ICU) admission, development of septic shock, and need for continuous renal replacement therapy).
Sample Processing and SARS-Cov-2 Virus Detection
SARS-CoV-2 RNA detection in nasopharyngeal swab was performed using RT-PCR targeted at open reading frame 1ab (ORF 1ab) and nucleocapsid protein (N) genes. RNA was extracted from the samples and then underwent real-time RT-PCR with SARS-CoV-2-specific primers and probes. A cycle threshold (Ct) value of less than 37 was defined as a positive test, and a Ct value of 40 or more was considered as a negative test. An equivocal result, defined as a Ct value between 37 and 40, required confirmation by retesting. If the repeated Ct value was less than 40 and an obvious peak was observed, or if the repeated Ct value was less than 37, the result was deemed positive.
Statistical Analysis
No statistical sample size calculation was done, and sample size was equal to the number of hospitalized patients with SARS-CoV-2 infection fulfilling inclusion criteria. Descriptive data were reported as median with interquartile ranges (IQR) or count and percentage, as appropriate.
Univariable and multivariate backward stepwise regression analyses were performed to evaluate the association between PVS and host factors and clinical outcome. Variables with p < 0.10 in the univariate models were candidates for multivariate analysis. Two-sided p values of 0.05 or less were considered as statistically significant. Data were retrieved from an online database for anonymous and automatic data collection [21]. The analyses were performed using SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA).
Results
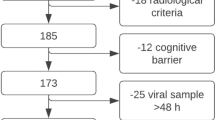
During the study period, 317 patients with SARS-CoV-2 infection were admitted to our hospital. Of those, 106 (33.4%) died within the first 21 days after diagnosis without evidence of SARS-CoV-2 RNA clearance, 82 (25.8%) were discharged early (less than 21 days from diagnosis) into fiduciary isolation and 8 (2.5%) had missing virological data. The remaining 121 patients fulfilled the inclusion criteria and were included the present study (Fig. 1). Clinical characteristics, treatments, and main outcomes of our study population are shown in Table 1.
Cumulative Incidence and Factors Associated with PVS
Overall, the definition of PVS (21 days or more) was fulfilled by 46/121 patients (38.0%), of whom 33 (27.3%), 15 (12.3%), and 5 (4.1%) continued to present viral shedding on day 28, day 35, and day 42, respectively. The cumulative incidence of patients with positive SARS-CoV-2 PCR at different time points is shown in Fig. 2.
Comparison between patients with PVS and those without PVS is shown in Table 2. According to univariate analysis, factors associated with PVS were immunosuppression (6.7% vs 21.7%, p = 0.02), increased IL-6 levels at the time of diagnosis (43.4% vs 67.3%, p = 0.02), time from onset of symptoms to diagnosis (median days 7.0 vs 3.5, p = 0.001), ICU admission (22.7% vs 43.5%, p = 0.02), and need for invasive mechanical ventilation (20.0% vs 41.3%, p = 0.013). PVS was not associated with antiviral treatment and anti-inflammatory therapy (corticosteroids or tocilizumab). The multivariate analysis confirmed immunosuppression, increased IL-6 levels at the time of diagnosis, time from onset of symptoms to diagnosis, and need for invasive mechanical ventilation as independent factors associated with PVS.
Discussion
In our study population of hospitalized patients with SARS-CoV-2 infection, we found that persistent viral shedding is common and associated with immunosuppression and increased IL-6 at the time of diagnosis, as well as time from onset of symptoms to diagnosis and the need for mechanical ventilation.
The present study analyzed a large database of prospectively collected data concerning hospitalized patients with SARS-CoV-2 infection in a university tertiary care hospital in Italy. To our knowledge, it is also the largest European prospective observational study analyzing SARS-CoV-2 RNA shedding using homogenous specimens (nasopharyngeal swabs), providing a unique opportunity to understand the clinical significance of viral shedding in these patients. Data were collected according to daily clinical practice, making our findings representative for the current management of patients with infections due to SARS-CoV-2.
The lack of a commonly accepted definition makes it difficult to address the issue of PVS in SARS-COV-2 infection [13,14,15,16]. Some authors considered PVS as the detection of SARS-CoV-2 RNA more than 15 days after symptoms [15]. Others required a longer period of time (e.g., more than 24 days) [16]. Our definition of PVS is more consistent with the recent literature reporting SARS-CoV-2 RNA detection for a median of 21 days after symptom onset [8, 9, 14]. Using this specific time point, we found that up to 38% of hospitalized patients with SARS-CoV-2 infection experienced PVS, which is within the 32.7–48.2% range of PVS reported in other series [15, 16]. However, we are not sure whether PVS means that virus is viable and can be transmitted. Indeed, SARS-CoV-2 infectiousness may significantly decline 8–18 days after symptoms onset, as suggested by previous studies in which live virus could no longer be cultured despite concomitant positive PCR results [12, 22]. Future investigations analyzing the potential for transmission in patients with PVS are required.
The clinical significance of PVS in patients with SARS-COV-2 remains unclear. Previous studies related PVS to male sex [15], older age [13, 14, 16], or chronic comorbidities [16]. In our study population, we did not find any correlation between PVS and age or sex, although immunosuppression significantly increased the risk of PVS sevenfold (p = 0.019). This observation may highlight individuals who need to be closely monitored or on whom treatment interventions should be focused in the future.
Interestingly, we found a higher probability of PVS among patients with increased levels of IL-6 at the time of diagnosis. This result is consistent with recent experimental models suggesting that increased IL-6 levels during antiviral immune responses can negatively impact the lytic activity of cytotoxic T lymphocytes [23], thus promoting viral persistence. Ours is the first study to show this association in SARS-CoV-2 infection. This finding may point to the complex role of immunomodulators in the treatment of patients with SARS-COV-2 infection [24].
We also found that PVS was mainly observed in patients with a more severe clinical course of SARS-CoV-2 infection. This was also so in previous studies, with the need for mechanical ventilation being 9.8 times more frequently in patients with persistent viral shedding when compared with those presenting faster conversion [15].
We believe that our results represent a significant step forward in better management of patients with SARS-CoV-2 infection. As a matter of fact, a more stringent approach to infection control may be necessary for immunosuppressed patients, including strict droplet precautions and isolation for an extended period of time than that currently recommended (14 day) [25]. Moreover, since the duration of viral shedding is often used as an endpoint for clinical trials evaluating new antivirals [26], we suggest a stratification based on underlying comorbidities or IL-6 levels in order to avoid imbalances between treatment arms.
This study has some limitations that should be assessed. First, we excluded from our study population those patients who early died or were discharged early. Accordingly, the percentage of patients with PVS may have been different if we could have obtained information about all patients consecutively hospitalized for SARS-CoV-2 infection at our hospital. Second, we did not analyze the viral load; therefore, quantification methods could impact our conclusions. Third, because we did not perform daily collection of nasopharyngeal swabs we were not able to estimate the overall duration of viral shedding. Fourth, we only included hospitalized patients with severe SARS-CoV-2 infection; accordingly, our findings could not be extended to patients with mild or moderate disease who were not admitted to the hospital or discharged early.
Conclusion
PVS is a common finding in hospitalized patients with SARS-CoV-2 infection, and is associated with immunosuppression and the need for mechanical ventilation. In our opinion, future studies should focus on the unknown meaning of this phenomenon in terms of infectivity and infection control measures.
References
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Invest. 2020;50(3):e13209.
Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.
Vena A, Berruti M, Adessi A, et al. Prevalence of antibodies to SARS-CoV-2 in Italian adults and associated risk factors. J Clin Med. 2020;9(9):2780.
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13.
Vena A, Giacobbe DR, Di Biagio A, et al. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect. 2020;26(11):1537–44.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
He X, Lau EHY, Wu P, , et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–5.
Li N, Wang X, Lv T. Prolonged SARS-CoV-2 RNA shedding: not a rare phenomenon. J Med Virol. 2020;92(11):2286–7.
Yang JR, Deng DT, Wu N, Yang B, Li HJ, Pan XB. Persistent viral RNA positivity during recovery period of a patient with SARS-CoV-2 infection. J Med Virol. 2020;92(9):1681–3.
Liu WD, Chang SY, Wang JT, et al. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect. 2020;81(2):318–56.
Hu X, Xing Y, Jia J, et al. Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci Total Environ. 2020;728:138812.
Xiao AT, Tong YX, Gao C, Zhu L, Zhang YJ, Zhang S. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clin Virol. 2020;127:104346.
Xu K, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis. 2020;71(15):799–806.
Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis. 2020;71(16):2249–51.
Zheng X, Chen J, Deng L, et al. Risk factors for the COVID-19 severity and its correlation with viral shedding: a retrospective cohort study. J Med Virol. 2020. https://doi.org/10.1002/jmv.26367.
Li TZ, Cao ZH, Chen Y, et al. Duration of SARS-CoV-2 RNA shedding and factors associated with prolonged viral shedding in patients with COVID-19. J Med Virol. 2020. https://doi.org/10.1002/jmv.26280.
Zuo Y, Liu Y, Zhong Q, Zhang K, Xu Y, Wang Z. Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID-19: a retrospective study in two designated hospitals in Anhui, China. J Med Virol. 2020;92(11):2666–74.
Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382(19):1787–99.
Giannini B, Riccardi N, Cenderello G, Di Biagio A, Dentone C, Giacomini M. From Liguria HIV web to Liguria infectious diseases network: how a digital platform improved doctors’ work and patients’ care. AIDS Res Hum Retroviruses. 2018;34(3):239–40.
Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–9.
Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The role of interleukin 6 during viral infections. Front Microbiol. 2019;10:1057.
Bassetti M GD, Aliberti S, Barisione E, et al. Balancing evidence and frontline experience in the early phases of the COVID-19 pandemic: current position of the Italian Society of Anti-Infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). Clin Microbiol Infect. 2020;26(7):880–94.
European Centre for Disease Prevention and Control. Guidance for discharge and ending isolation in the context of widespread community transmission of COVID-19 – first update. ECDC, Stockholm. 8 Apr 2020. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-guidance-discharge-and-ending-isolation-first%20update.pdf. Accessed 10 June 2020.
Wei Tang ZC, Mingfeng H, Zhengyan W, et al. Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomized, controlled trial. medRxiv. 2020. https://doi.org/10.1101/2020.04.10.20060558.
Acknowledgements
We thank the participants of the study. The authors acknowledge as coauthors all the members of the GECOVID-19 Study group.
Funding
No funding or sponsorship was received for this study or the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
List of Investigators
GECOVID-19 Study group: Anna Alessandrini, Marco Camera, Emanuele Delfino, Andrea De Maria, Chiara Dentone, Antonio Di Biagio, Ferdinando Dodi, Antonio Ferrazin, Giovanni Mazzarello, Malgorzata Mikulska, Laura Nicolini, Federica Toscanini, Daniele Roberto Giacobbe, Antonio Vena, Lucia Taramasso, Elisa Balletto, Federica Portunato, Eva Schenone, Nirmala Rosseti, Federico Baldi, Marco Berruti, Federica Briano, Silvia Dettori, Laura Labate, Laura Magnasco, Michele Mirabella, Rachele Pincino, Chiara Russo, Giovanni Sarteschi, Chiara Sepulcri, Stefania Tutino (Clinica di Malattie Infettive); Roberto Pontremoli; Valentina Beccati; Salvatore Casciaro; Massimo Casu; Francesco Gavaudan; Maria Ghinatti; Elisa Gualco; Giovanna Leoncini ; Paola Pitto; Kassem Salam (Clinica di Medicina interna 2); Angelo Gratarola; Mattia Bixio; Annalisa Amelia; Andrea Balestra; Paola Ballarino; Nicholas Bardi; Roberto Boccafogli; Francesca Caserza; Elisa Calzolari; Marta Castelli; Elisabetta Cenni; Paolo Cortese; Giuseppe Cuttone; Sara Feltrin; Stefano Giovinazzo; Patrizia Giuntini; Letizia Natale; Davide Orsi; Matteo Pastorino; Tommaso Perazzo; Fabio Pescetelli; Federico Schenone; Maria Grazia Serra; Marco Sottano (Anestesia e Rianimazione; Emergenza Covid padiglione 64 “Fagiolone”); Roberto Tallone; Massimo Amelotti; Marie Jeanne Majabò; Massimo Merlini; Federica Perazzo (Cure intermedie); Nidal Ahamd; Paolo Barbera; Marta Bovio ; Paola Campodonico; Andrea Collidà; Ombretta Cutuli; Agnese Lomeo; Francesca Fezza Nicola Gentilucci; Nadia Hussein; Emanuele Malvezzi; Laura Massobrio; Giula Motta; Laura Pastorino; Nicoletta Pollicardo; Stefano Sartini; Paola Vacca Valentina Virga (Dipartimento di Emergenza ed accettazione); Italo Porto; Giampaolo Bezante; Roberta Della Bona; Giovanni La Malfa; Alberto Valbusa; Vered Gil Ad (Clinica Malattie Cardiovascolari); Emanuela Barisione; Michele Bellotti; Teresita Aloè; Alessandro Blanco; Marco Grosso; Maria Grazia Piroddi; Maria Grazia Piroddi (Pneumologia ad Indirizzo Interventistico); Paolo Moscatelli; Paola Ballarino; Matteo Caiti; Elisabetta Cenni; Patrizia Giuntini Ottavia Magnani (Medicine d’Urgenza); Samir Sukkar; Ludovica Cogorno; Raffaella Gradaschi; Erica Guiddo; Eleonora Martino; Livia Pisciotta (Dietetica e nutrizione clinica); Bruno Cavagliere; Rossi Cristina; Farina Francesca (Direzione delle Professioni sanitarie); Giacomo Garibotto; Pasquale Esposito (clinica nefrologica, dialisi e trapianto); Giovanni Passalacqua; Diego Bagnasco; Fulvio Braido; Annamaria Riccio; Elena Tagliabue (Clinica Malattie Respiratorie ed Allergologia); Claudio Gustavino; Antonella Ferraiolo (Ostetricia e Ginecologia); Salvatore Giuffrida; Nicola Rosso (Direzione Amministrativa); Alessandra Morando, Riccardo Papalia; Donata Passerini; Gabriella Tiberio (Direzione di presidio); Giovanni Orengo; Alberto Battaglini (Gestione del rischio clinico); Silvano Ruffoni; Sergio Caglieris.
Disclosures
Matteo Bassetti reports grants and personal fees from Pfizer, grants and personal fees from MSD, grants and personal fees from Cidara, personal fees from Astellas, personal fees from Gilead. DRG reports personal fees from Stepstone Pharma GmbH and an unconditional grant from MSD Italia. Matteo Bassetti is also a member of the journal’s editorial board. Antonio Vena, Lucia Taramasso, Antonio Di Biagio, Malgorzata Mikulska, Chiara Dentone, Andrea De Maria, Laura Magnasco, Laura Ambra Nicolini, Bianca Bruzzone, Giancarlo Icardi, Andrea Orsi, Paolo Pelosi, Lorenzo Ball, Denise Battaglini, Iole Brunetti, Maurizio Loconte, Nicolò A. Patroniti, Chiara Robba, Martina Bavastro, Matteo Cerchiaro, Daniele Roberto Giacobbe, Irene Schiavetti and Marco Berruti have nothing to disclose.
Compliance with Ethics Guidelines
The study was carried out in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of Liguria Region (N. CER Liguria 114/2020 - ID 10420). All patients provided verbal informed consent because of isolation precautions for performing nasopharyngeal swab according to the local protocol approved by hospital authorities, for data collection and for participation in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Vena, A., Taramasso, L., Di Biagio, A. et al. Prevalence and Clinical Significance of Persistent Viral Shedding in Hospitalized Adult Patients with SARS-CoV-2 Infection: A Prospective Observational Study. Infect Dis Ther 10, 387–398 (2021). https://doi.org/10.1007/s40121-020-00381-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-020-00381-8