Abstract
When taken consistently, pre-exposure prophylaxis (PrEP) against human immunodeficiency virus (HIV) with once daily tenofovir disoproxil fumarate-emtricitabine (TDF-FTC) has been shown to safely reduce the incidence of HIV infection in high-risk individuals by more than 90%. Yet, according to the Centers for Disease Control and Prevention, there were about 2.1 million new cases of HIV reported worldwide in 2015. Undoubtedly, there is significant room for improvement to prevent the transmission of HIV. Research to date has been heavily focused on the high-risk men who have sex with men (MSM) population, yet, many women worldwide remain at high risk of HIV transmission. PrEP offers women a protection method that is discrete, does not require partner consent, and may be compatible with both contraception or conception as desired. However, women often remain under-represented in HIV prevention literature and are reported to have lower real-world uptake in comparison to men. Furthermore, clinical trials that do focus on the female population demonstrate mixed efficacy results that highlight the adherence challenges in this population. It is essential to identify factors that contribute to PrEP non-adherence as well as barriers to preventative treatment. This review will discuss the clinical evidence behind PrEP in women, current barriers to use afflicting this population, pharmacotherapy considerations for the female patient, alternative and future agents, and the current real-world application of PrEP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pre-exposure prophylaxis (PrEP) is the use of antiretroviral (ARV) drugs to prevent human immunodeficiency virus (HIV) acquisition in HIV-negative individuals at high risk of acquiring the virus [1]. It is an important adjunctive strategy to reduce HIV transmission in combination with condom use, counseling, and early diagnosis and treatment. It is recommended by the World Health Organization (WHO) for the prevention of HIV transmission in persons at substantial risk of developing HIV infection, as defined by incidence rates greater than or equal to 3 per 100 person-years [1]. After the WHO published the initial guidance on PrEP use in July 2012, the United States (US) Food and Drug Administration (FDA) became the first agency to approve the drug combination tenofovir disoproxil fumarate-emtricitabine (Truvada®; TDF-FTC) for this indication [2]. Subsequently, the US Public Health Service (USPHS) and Centers for Disease Control and Prevention (CDC) released the first comprehensive guideline in May 2014 [3]. Several other countries, such as France, Canada, and South Africa, have also since approved Truvada® for PrEP, as significant interest and research have mounted regarding this HIV prevention strategy [4]. A brief summary of the major PrEP guidelines, including the most recent WHO update and European Acquired Immune Deficiency Syndrome (AIDS) Clinical Society recommendations, is provided in Table 1.
Women represent a unique and important population to consider in the evolution of PrEP. As of 2015, the WHO and Joint United Nations Programme on HIV/AIDS (UNAIDS) estimate approximately 17.8 million women over the age of 15 are living with HIV across the globe [5]. Females aged 15–24 years are twice as likely to be at risk of HIV infection compared with aged-matched male counterparts worldwide, a difference related to unsafe and unwanted sexual activity, and further enhanced by racial and geographical disparities. HIV/AIDS is also the leading cause of death worldwide in females aged 15–44 years [6]. Reducing HIV infection rates in women of all ages and backgrounds is crucial for disease control. However, many social factors, such as distrust associated with condom use, lack of communication between men and women regarding sexual health issues, and overall poor perception of HIV vulnerability, significantly limit the ability of many women to practice safer sex [7]. Unlike most other HIV prevention strategies, PrEP serves as a highly effective method that women can utilize without requiring negotiation, commitment, or consent from their partner(s). Despite such advantages, the overall utilization of PrEP by women remains low. This review summarizes important data relevant to the efficacy, safety, real-world application, and future of PrEP as it relates to the female population. From inception to April 2017, a comprehensive electronic search was conducted using PubMed, Embase, and MEDLINE to identify relevant studies for all elements of this review. Systematic reviews, meta-analyses, randomized controlled trials, and pharmacokinetic studies were specifically targeted. Reference lists of relevant articles were examined to identify further studies for inclusion. Focus was placed on trials published from 2016 to present. Abstracts and oral presentations from recent research proceedings were manually screened for appropriate content. Additionally, clinicaltrials.gov and the HIV Prevention Trials Network (HPTN) websites were searched for ongoing PrEP studies. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Female Representation in PrEP Literature and the Impact of Nonadherence
The USPHS/CDC recommend PrEP use in three targeted populations: men who have sex with men (MSM), heterosexual women and men, and people who inject drugs (PWID) who are at substantial risk of HIV acquisition [3]. Interestingly, two of the pivotal trials to support PrEP implementation, the iPrEx study and the US MSM Safety Trial, only included men or transgender women who have sex with men [8, 9]. Of the five published trials summarized in the CDC guidelines that included women, the data were mixed.
The Partners PrEP trial studied heterosexual HIV-1 serodiscordant couples in Uganda and Kenya, whereby the seronegative partner was female in 38% of couples (n = 1785). Treatment arms included TDF-FTC and TDF alone versus placebo. Among women, efficacy was 71% with TDF (p = 0.002) and 66% with TDF-FTC (p = 0.005) compared to placebo. No significant difference in efficacy was identified between sexes. When corrected for adherence and tenofovir (TFV) plasma concentrations, 86% and 90% risk reductions were achieved in both men and women receiving TDF and TDF-FTC, respectively [10]. The TDF2 study examined the use of daily TDF-FTC for PrEP among young adult heterosexual men and women in Botswana. Women accounted for 45.7% (n = 557) of the study population. Overall, PrEP resulted in a 63% reduction in the risk of HIV infection compared to placebo. However, seven infections occurred in women, translating to a non-significant risk reduction of only 49.4%. Although medication adherence, as measured by pill count and self-report, was generally high in the entire population, plasma drug monitoring demonstrated detectable TFV and FTC in 80% of subjects who did not seroconvert compared to only 50% of those who became infected [11].
Other trials performed solely in females have magnified the impact of adherence on PrEP efficacy in this population. The randomized, double-blind, placebo-controlled FEM-PrEP trial evaluated PrEP in women aged 18–35 years in Kenya, South Africa, and Tanzania. The study was stopped early because of a lack of benefit of TDF-FTC. Incidence of HIV-1 acquisition in the TDF-FTC group (n = 1062) was 4.7 per 100 person-years (events = 33) compared to 5.0 per 100 person-years in the placebo group (n = 1058) (events = 35) (p = 0.81). Of the 27 subjects receiving TDF-FTC who acquired HIV, target TFV plasma levels were achieved in only 7, despite a 95% self-reported adherence [12]. Similarly, the VOICE trial compared TDF alone, TDF-FTC, and a 1% tenofovir-containing vaginal microbicide gel versus placebo in 5029 women aged 18–45 years in South Africa, Uganda, and Zimbabwe. Both the TDF and gel groups were stopped early because of futility. Overall, 312 new seroconversions occurred, and no significant differences between any of the active treatment arms and their respective placebos were identified. Despite a 91% retention rate and high reported adherence, serum drug monitoring again demonstrated detectable TFV levels in less than half of the intended population [13].
One hypothesis for the early disappointing findings was that efficacy may be reduced in higher risk populations. A subgroup analysis of the Partners PrEP study sought to evaluate this possibility [14]. Subjects were identified using a risk-assessment tool based on factors such as number of children, unprotected sex, and plasma viral load of infected partner [15]. Of the 4733 participants, 1780 were women. The incidence of HIV in women receiving placebo was 6.6 per 100 person-years compared to 1.9 in the TDF group (efficacy = 69%, p < 0.04) and 2.4 in the TDF-FTC group (efficacy = 64%, p < 0.05). Monthly clinic adherence was >94% in all subgroups throughout the study with detectable TFV plasma concentrations in more than 70% of samples. This analysis demonstrated that in high-risk heterosexual women, oral PrEP with either TDF or TDF-FTC remained highly effective in preventing HIV when taken consistently; therefore, increased risk likely did not explain PrEP failure.
Lastly, the Bangkok Tenofovir Study evaluated the efficacy of TDF compared to placebo in preventing HIV-1 infections in male and female PWID. Approximately 20% of the study population were women (n = 489). Interestingly, this trial was associated with the highest rates of medication adherence (66%) by females, as evidenced by plasma drug concentrations. Efficacy in reducing HIV-1 acquisition was 78.6% in women [16]. Engagement by this cohort of PWID was further supported when 61% of eligible participants for the observational, open-label extension trial chose to pursue PrEP [17].
Overall, varying degrees of adherence reported throughout these studies have contributed to varying degrees of efficacy. Findings were confirmed by a recent meta-analysis comprising data from 18 PrEP studies including a pooled baseline population of 8714 women. An overall risk reduction of 70% was found in studies with high (>70%) adherence, whereas no protective effect was found in studies with low (≤40%) adherence. In this meta-analysis, no significant difference in efficacy among sexes was found [18]. The overarching message is that adherence is key and that engaging women in PrEP therapy may be particularly challenging.
Barriers to Appropriate PrEP Use
Health literacy as well as individual attitudes, values, preferences, and beliefs are all factors that may influence PrEP adherence, and thereby efficacy, among females [19]. From a global perspective, these factors may vary widely depending on geography and culture. American subjects in Philadelphia were recently surveyed to identify such barriers [20]. Participants underwent HIV testing in conjunction with interviews regarding attitudes toward PrEP and self-perceived infection risk. The cohort included a total of 2721 females; 64% were under the age of 35 and 90% self-identified as African American. A top reason cited by women for not using PrEP therapy was lack of perceived risk of acquisition. In fact, only 8.3% of females believed themselves to be at moderate or high risk of HIV infection compared to 56.8% of men. Interestingly, 9 of the 35 individuals who tested positive for HIV were females who did not perceive themselves to be at moderate or high risk. Four expressed disinterest in PrEP altogether. Overall, more men expressed openness to PrEP therapy than women (61.4% vs. 54.8%, respectively, p < 0.0001). On the contrary, young females surveyed in South Africa and Kenya indicated strong interest in PrEP [21]. Various secondary benefits were also cited by differing populations—female sex workers acknowledged they would feel safer in their practice, serodiscordant couples hoped that PrEP would invigorate their sex lives, and adolescent girls appreciated the privacy of personal use.
Survey-based studies of women in the US and Africa have also demonstrated that providing options regarding drug formulation improves openness to PrEP therapy [20, 22, 23]. The attitudes of women towards PrEP have been documented much like those related to contraception: women are seeking a choice between different routes of medication administration and formulations that are suitable for their daily lives. Acceptance also depends upon patient experiences, perceptions of product attributes, and use requirements. As alluded to previously, these personal preferences must also be considered within the context of sexual relationships, communities, beliefs, and culture that change with time [22]. In a follow-up study to the VOICE trial, 68 female participants engaged in in-depth interviews to explore PrEP preferences and reasons for nonadherence [22]. The majority of women preferred long-acting injectable, implantable, or vaginal ring formulations compared to oral tablets, vaginal films, suppositories, or gels. Factors driving participant preference included ease of adherence and administration, social stigma, and partner input. From these data, it is evident that a spectrum of preferences and individualized barriers exists among women worldwide; thereby, providing greater options may enhance PrEP marketing and improve the public health approach to decreasing transmission.
In addition to intrinsic differences in perceived risk, cultural pressures, and personal preferences, other commonly cited barriers to PrEP are concerns over safety, side effects, effectiveness, and cost [24]. A 2015 study by Auerbach and colleagues served to elucidate some of these barriers in a cross-sectional study of patients from an urban US clinic population [25]. This study specifically targeted African American women, who are often underrepresented and at higher risk for the transmission of HIV. Of the 144 women who participated in this study, 92% were black, 53% were single, divorced, or separated, 52% were employed, and 47% had annual incomes between $10,000 and $40,000. Identified barriers included concerns about cost and side effects, mistrust of medical institutions, social stigma, novelty of the medication and related efficacy/safety concerns, and lack of stable housing situations. Participants also shed light on other factors that may deter daily PrEP use, including depression and low self-esteem. This study highlights the need for additional psychosocial support to ensure success of PrEP therapy in certain populations. Additionally, it demonstrates that concerns among women may vary based on socioeconomic factors and education level. Targeted interventions to provide education about HIV risk and the efficacy and safety of PrEP may encourage American women to seek out preventative treatment [25]. A novel phenomenon that may shift female attitudes regarding PrEP is the concept of control over HIV acquisition. Unlike male condoms, the use of oral PrEP is now within the control of the woman, which may empower them to take advantage of this preventive strategy.
Truvada® Pharmacotherapy: Considerations for the Female Patient
Pharmacokinetics and Pharmacodynamics
PrEP efficacy is thought to be correlated with sustained active drug presence in anogenital tissues and fluids, the likely sites of initial HIV exposure. Thus, distribution and elimination half-life are two major pharmacokinetic (PK) concerns related to drug selection. Sex may influence these parameters because of biological differences such as surface area and cell types between men and women. Various studies have assessed these PK parameters to evaluate whether they may explain variability in HIV prevention by PrEP. Patterson and colleagues studied the decay of TFV and FTC and their active metabolites in plasma, genital fluids, and genital mucosal tissues. Blood plasma concentrations of TFV and FTC were quantifiably detectable in 50% of patients up to 14 days after a single oral dose was administered to 15 healthy individuals, including 7 females [26]. The terminal elimination half-lives for TDF and FTC were 47 and 49 h, respectively, calculated from 7 to 14 days using a sensitive assay. Although this finding would suggest inherently high HIV protection in the PrEP trials, tissue concentrations achieved in the female genital tract tell a different story. In the same study, rectal tissue concentrations of TFV and TFV diphosphate (DP) were found to be 100-fold higher than those of vaginal and cervical tissue. On the other hand, FTC concentrations were 10- to 15-fold higher in vaginal and cervical tissue than in rectal tissue. Differences in tissue penetration alludes to the idea that customary dosing regimens of TDF may not produce adequate concentrations in the female genital tract for the purposes of protection against acute HIV infection and may help explain variable PrEP efficacy by sex. Several other studies have concluded similar results, including an intensive 60-day PK study of daily TDF-FTC in both HIV-positive and seronegative adults (13 total females) [27,28,29]. The authors reported ten-time higher drug accumulation in rectal mononuclear cells compared to other cell types. This study also suggested that while minor differences in first dose kinetics may exist, TDF and FTC pharmacology appeared similar for both prevention and treatment [29].
Some studies have attempted to link PK findings to dosing principles. Cottrell and colleagues utilized a model evaluating colorectal and genital mucosal concentrations of TFV, FTC, and their active metabolites in 47 healthy women [30]. Colorectal concentrations of TFV-DP were again ten times higher than in the lower female genital tract. This model predicted that 6–7 doses per week would be required to provide protection against HIV in the lower female genital tract compared to 2 doses per week to provide colorectal tissue protection. It is important to note, however, that such PK analyses are limited by subject demographics, sample size, dosing regimens, in vitro and ex vivo methodologies and cannot clearly link results with actual HIV acquisition rates.
Other PK properties of ARVs that may affect PrEP efficacy have not been fully elucidated. It has been suggested that agents with low protein-binding capacity, such as FTC, attain higher tissue concentrations than those that are highly protein-bound (e.g., lopinavir) [28]. Furthermore, the long plasma elimination half-lives of certain agents may contribute to the development of viral resistance, hindering the safety of these agents for prophylaxis [31]. Future research should also focus on unexplored concepts such as the impact of inflammation or tissue damage on PrEP efficacy, the impact of ARV metabolites in curbing infection rates, and the ability of ARVs to permeate specific types of tissues (e.g., mucosal vs. lymphoid).
Safety
The two most well-known toxicities of TDF include renal dysfunction and bone demineralization. While extensive data regarding the safety of TDF-FTC in the HIV-infected population exist, data specific to various populations of PrEP users may not be interchangeable. To this aim, a per-protocol safety analysis examining the changes in estimated glomerular filtration rates (eGFR) of participants in the Partners PrEP study was performed. TDF-based PrEP was associated with a small but significant decline in eGFR, which appeared by 4 weeks, remained stable to 12 months, and gradually weaned thereafter. The authors concluded that the non-progressive decline in eGFR was not associated with a substantial increase in the risk of clinically relevant eGFR decline. No differences in age or sex were seen [32]. Renal and hepatic safety data of TDF-FTC from the FEM-PrEP trial were also separately evaluated. This analysis did not find a statistically significant difference in renal toxicity compared to placebo. Women randomized to TDF-FTC did have significantly higher rates of asymptomatic, mild to moderate elevations in AST and ALT, particularly in women with prior hepatitis B virus exposure. However, a definitive association could not be determined [33].
Bone mineral density (BMD) data are sparser in the female versus male PrEP populations. BMD was assessed in a subset of participants from the TDF2 trial, which included 54 women receiving TDF-FTC and 60 women receiving placebo. T and z scores were significantly lower in the forearm, hip, and lumbar spine of the treatment groups, but no difference in fracture incidence occurred [11]. Similar findings were observed in the male cohorts, as well as the data from the iPrEX trial and a US MSM subset study [8, 11, 34]. BMD reductions were less pronounced in other placebo-controlled trials [8, 10, 12]. As such, the CDC does not recommend routine screening prior to starting PrEP, though high-risk individuals may warrant testing. Fortunately, the overall safety data regarding drug toxicity remain promising. It is, however, prudent to caution that low adherence rates and limited study durations may reduce generalizability.
Beyond adverse drug reactions, safety related to emerging drug resistance has been a concern since the inception of PrEP. While a complete exploration of ARV resistance exceeds the scope of this review, studies do document identification of resistance mutations, often M184I/V or K65R, most commonly in subjects with unrecognized acute HIV infection at the time of study drug initiation [3]. However, there have been isolated reports of resistance in subjects seroconverting on PrEP, even during confirmed drug adherence [35]. To our knowledge, all cases have been in males. While thus far, resistance has been uncommon, the individual and public health impact of real-world use is unknown. Such findings emphasize the importance of close patient monitoring and continued investigation into ARV resistance.
Reproductive Considerations
Minimizing the risk of HIV acquisition during times of desired conception is important. Traditional options for discordant couples include sperm washing, in vitro fertilization or intrauterine insemination, or treatment as prevention in the HIV positive partner [36]. Accessibility and financial limitations can limit these strategies. PrEP is also now a viable alternative, despite that in most PrEP trials, therapy was stopped if pregnancy was detected. However, in a 2017 systematic review, Mofenson and colleagues examined the safety of TDF in both HIV-infected and uninfected women during pregnancy and lactation [37]. Overall, the authors found no significant difference in pregnancy incidence or loss, preterm delivery less than 37 weeks, low birth weight (less than 2500 g), birth defects, or infant or maternal mortality between TDF and non-TDF regimens (zidovudine/single-dose nevirapine), including placebo. In trials of HIV-uninfected women specifically, there were no differences seen between women receiving TDF or placebo in terms of preterm delivery, low birth weight, birth defects, and neonatal/infant mortality at 12 months. Zero maternal deaths were reported in the studies of HBV-monoinfected women. Rates of both preterm delivery and low birth weight were lower in HIV-uninfected women compared with HIV-infected women. The authors concluded that the lack of TDF impact on maternal and infant safety outcomes suggests an overall net benefit of PrEP in high-risk women during pregnancy and lactation [37].
Additional safety data for TDF-FTC use during lactation come from a study of five HIV-infected, breastfeeding mothers that found lower drug exposure from breast milk compared with exposure in utero [38]. Similarly, in an open-label study of 50 HIV-uninfected African breastfeeding women given directly observed oral PrEP, infant plasma concentrations of TDF and FTC were 12,500-fold and 200-fold lower than what are expected from pediatric treatment doses for vertical HIV prophylaxis. The authors thereby concluded that PrEP can be safely used during breastfeeding [39]. Overall, while findings are promising, the data are limited and the decision for PrEP use in pregnant or breastfeeding women should be made on an individual basis between the patient and healthcare provider.
Alternative PrEP Strategies and Future Directions
The ideal drug for PrEP is one that is safe, efficacious, achieves high concentrations in targeted tissues, maintains a high barrier to resistance, and is convenient in terms of dosing, cost, and accessibility [40]. TDF-FTC fits this profile in several ways. However, given that the dual-nucleos(t)ide reverse transcriptase inhibitor (NRTI) combination is a cornerstone of most initial HIV treatment regimens, potential resistance is a significant concern. Furthermore, formulations beyond oral pills and less frequent dosing regimens are desirable as evidenced by aforementioned studies. Therefore, consideration of other ARV classes for the purpose of PrEP is warranted. Recent literature evaluating various other PrEP strategies is summarized below, with additional trial highlights in Table 2.
Nucleoside Reverse Transcriptase Inhibitors
Tenofovir alafenamide (TAF) is a prodrug of tenofovir that, unique to its predecessor TDF, is metabolized into the active compound tenofovir-diphosphate (TFV-DP) intracellularly rather than in blood [41]. This results in higher tissue concentrations of active drug and lower plasma concentrations and is thus associated with an improved safety profile.
While the safety and efficacy of TAF in the treatment of HIV have been well established, TAF has not been as extensively studied as PrEP. Initially, oral TAF-FTC was investigated in macaques given repeated rectal challenges against simian-HIV (SHIV). While results suggested that rectal exposure to the active drug may be reduced, the combination was 100% efficacious (zero infections following 19 exposures) and was considered feasible for human PrEP [42]. A long-acting, subdermal implant containing TAF has also been studied in dogs. A device delivering 0.92 mg daily of TAF produced sustained plasma levels of TAF and its active metabolite [43]. Similarly, a tunable thin-film polymer device containing TAF has been developed as a biodegradable, subcutaneous implant device for PrEP [44]. An initial in vitro study of the TAF-based device demonstrated that when co-formulated with PEG300 to increase dissolution and solubility, target drug release rates were achieved. Furthermore, by “tuning” the device’s membrane thickness or surface area, the manufacturers are able to manipulate the duration or size of the implant without compromising release rates. These methodologies, with further study, could offer long-acting, user-independent delivery of TAF, which could be desirable for many PrEP users.
Recent TAF PrEP data in human subjects have highlighted an important point in that PK parameters suggesting efficacy of prophylactic regimens have not been fully validated. PK data from a study of eight healthy women following a single oral dose of TAF (25 mg) resulted in TFV-DP levels that were significantly lower in cervicovaginal fluid and genital/rectal tissues after TAF administration compared to levels expected with a 300 mg oral dose of TDF [45]. In addition, 83% of tissues had undetectable levels of TFV-DP. Studies linking biological markers with PrEP efficacy must take precedence before firm conclusions can be drawn. Nonetheless, an ongoing phase 3 study of TAF-FTC versus TDF-FTC vs. dual placebo is underway in uninfected MSM and transgender women [46].
Beyond TAF, a novel NRTI, 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) has also been developed and identified as a potential (distant) future PrEP candidate. It has greater potency and a longer intracellular half-life compared to other approved NRTIs [47]. It also displays activity against the K65R mutation, associated with TFV. Based on findings from a preclinical in vivo model in humanized mice, this agent was efficacious in preventing oral and vaginal HIV-1 transmission with a low toxicity profile, supporting further clinical development [48].
Chemokine Co-Receptor Type 5 Antagonist
Maraviroc (MVC), a chemokine co-receptor type 5 (CCR5) antagonist that impedes HIV entry into cells, is less commonly used in the treatment of HIV and is associated with low frequency viral resistance [40]. It also concentrates in the female genital tract [49]. It has been introduced in gel, ring, and oral formulations for PrEP. However, animal studies yielded mixed efficacy results, and in an ex vivo challenge in human rectal mucosa, it did not provide a protective effect against HIV [50,51,52]. Nonetheless, continued interest prompted studies examining MVC in combinations with TDF as an intravaginal ring in an ovine model, as well as oral formulations alone and in combinations with TDF and FTC in a phase 2 trial of MSM [53, 54]. In a recent prospective, randomized, double-blinded study involving uninfected females (n = 188) in the US, MVC was well tolerated, and no new HIV infections developed during the 48-week study period [55]. Additional evidence on this agent will be garnered when results from the Novel Exploration of Therapeutics (NEXT) for Pre-Exposure Prophylaxis (PrEP) study of males and females in the US and Puerto Rico become available [56].
Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
Rilpivirine (RPV), a second-generation NNRTI, has been developed as a long-acting (LA) nanosuspension injectable (TMC278 LA). Single- and multiple-dose phase I studies performed in HIV-negative men and women demonstrated that intramuscular (IM) administration of RPV produced rapid and persistent plasma and genital tract concentrations [57, 58]. However, the phase 1 MWRI-01 study showed concerning results for women. In this study (n = 36; 24 women) participants were assigned to one of two RPV injections [59]. Plasma levels, genital and rectal fluids, and tissue samples were tested for PK analysis. Investigators found the rectal tissue-to-plasma ratio was twofold higher than those of vaginal and cervical tissues. Likewise, sustained viral suppression was observed in rectal tissue but not cervical or vaginal tissue. The ongoing HPTN 076 is a phase 2 multi-site, double-blind study taking place in South Africa, Zimbabwe, and the US [60, 61]. Participants are randomized 2:1 to 1200 mg of RPV LA injections every 8 weeks (six total injections) or placebo for the assessment of safety and acceptability in low-risk, sexually active, HIV-uninfected women. Overall, 94% of the current study population is black, and the mean participant age is 31 years. Preliminary results demonstrate no statistical difference in observed adverse events between groups. In terms of acceptability, the majority (80%) of participants felt the injectable was easier to use, and 68% of women strongly agreed that they would definitely use an injectable form of PrEP in the future if available. Despite these favorable results, this study is not assessing efficacy. Due to the lack of efficacy evidence in tissue explant studies and storage feasibility related to the formulation's need for cold chain distribution and light protection, it is unclear whether RPV LA will progress to phase 3 PrEP trials [59]. Additionally, although LA formulations are ideal for improvement of PrEP, concerns exist regarding difficulty managing side effects and possible development of resistance if acute infection occurs at the tail end of a dosing period [62].
Dapivirine is an NNRTI initially developed as an oral ARV, yet was ultimately pursued as a microbicide [63]. Microbicides permit discreet, female-controlled prevention of sexually transmitted infections (STIs) and represent another major advancement that has infiltrated the PrEP pipeline. Previously, focus was centered on tenofovir vaginal gel, as implemented in the VOICE trial, but results were disappointing [13, 64]. Attention has now turned to the dapivirine intravaginal ring, developed by the International Partnerships for Microbicides (IPM), using similar technology as various hormonal products [65]. Vaginal rings are designed to be easily inserted by the female, fit comfortably in the vagina and produce sustained local microbicide delivery.
Two major phase 3 studies have demonstrated the ability of dapivirine to safely prevent HIV infection in over 4500 women in southern and eastern Africa [66, 67]. Both were randomized, double-blind, placebo-controlled trials utilizing a 25-mg dapivirine vaginal ring renewed once monthly in conjunction with counseling, support services, and free condoms. The ASPIRE trial included 2629 women aged 18–45 years (median 26 years) who were followed for a median of 1.6 years [66]. Overall, 71 incidental HIV-1 infections occurred in the dapivirine group and 97 in the placebo group, equating to a 27% reduced HIV incidence. While significant, protection was lower than hypothesized. Results differed according to age, which was also correlated with adherence in post hoc analyses. Efficacy was 61% (95% CI 32–77, p < 0.001) among women at least 25 years old and 10% (95% CI −41 to 43, p = 0.64) among women younger than 25 years. This highlights the challenges of HIV protection in young women. Notably, adverse events and STI rates were similar between groups.
The Ring study, led by Nel and colleagues, enrolled 1959 HIV-negative women aged 18–45 years [67]. Seroconversion rates were 4.1 per 100 person-years in the dapivirine group (n = 1307) and 6.1 per 100 person-years in the placebo group (n = 652). The corresponding overall seroconversion rate was 31% lower in the dapivirine group compared to the placebo group (HR 0.69, 95% CI 0.49–0.99; p = 0.04). Plasma and used-ring drug monitoring indicated that most subjects adhered to therapy, although limitations in such measures of adherence were acknowledged. Overall adverse event rates were similar between groups, though the number of serious adverse events was higher in the dapivirine group (2.9% vs. 0.9%; p = 0.008). Patterns to suggest clinical significance of difference in adverse event rates between groups were not identified. Furthermore, no adverse event was thought to be due to the product itself. Future research related to safety and efficacy in pregnant females, concomitant use of contraceptives, and NNRTI resistance are on the horizon as presented at the 2017 Conference on Retroviruses and Opportunistic Infections [68,69,70].
Integrase Strand Transfer Inhibitors
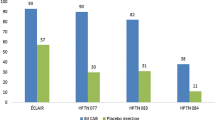
The integrase strand transfer inhibitor (INSTI) class agent with the greatest forward progress for PrEP is cabotegravir, a new agent similar in structure to dolutegravir. Its long elimination half-life is promising for extended interval dosing, which may prove useful for PrEP as well as HIV maintenance therapy [71]. Both oral and long-acting injectable formulations at various dose ranges and intervals have been explored. Early human PK studies demonstrated that single doses of cabotegravir (GSK1265744) with or without LA RPV produced therapeutic concentrations for ≥30 days [72, 73]. Positive results led to the initiation of two phase 2 clinical trials: the recently completed ECLAIR study in uninfected men and the ongoing HPTN077 trial in men and women [71]. The HPTN077 trial is planning to enroll approximately 60% women aged 18–65 years at low-to-minimal risk of HIV infection in the US, South America, and sub-Saharan Africa [74]. Additionally, a trial is underway that compares injectable cabotegravir to daily TDF-FTC for PrEP in HIV-uninfected MSM and transgender women who have sex with men, expected to be completed in 2020 [75]. While the early data appear encouraging, greater exploration into the efficacy of cabotegravir in broader populations may offer more flexibility for patients seeking PrEP. Also of interest are the studies of this agent in combination with RPV for HIV treatment, allowing for NRTI and protease inhibitor sparing regimens [73, 76].
On-Demand PrEP
Beyond recent research with new agents and formulations, another interesting concept is “on-demand” (or nondaily) PrEP. The IPERGAY trial assessed the efficacy of on-demand PrEP in high-risk uninfected MSM by administering TDF-FTC as a loading dose of two pills within two to 24 h prior to sexual activity, followed by a third pill 24 h after the loading dose, and a fourth pill 24 h later [77]. The authors’ hypothesized that adherence, and consequently efficacy, may be higher with this strategy. In the event of multiple consecutive sexual encounters, participants were instructed to continue taking one pill per day until their last sexual encounter and then two additional post-exposure pills. Two individuals developed HIV infection in the TDF-FTC arm, whereas 14 developed infections in the placebo arm (95% CI 40–98; p = 0.002). Although on-demand PrEP with TDF-FTC provided protection against HIV-1 in MSM, more data need to be collected to address the limitations of this small study. For example, the study participants took an average of 16 pills per month, which reflects a level of protection that is not akin to taking TDF-FTC for infrequent sex. Furthermore, evidence to support this practice in the female population is lacking, and in an aforementioned PK/pharmacodynamic modeling simulation, it was unfavorably estimated that women would require nine post-coital daily doses of TDF-FTC to match the efficacy in IPERGAY [30].
Real-World Application
Data regarding the efficacy of PrEP in women beyond the arena of controlled trials are also more limited in comparison with males. The PROUD study was an open-label, randomized trial in sexual health clinics in England [78]. In this all-male group, those offered immediate PrEP inferred a relative risk reduction of 86% (90% CI, p = 0.0001), corresponding to a total of 13 men needing to access PrEP for 1 year to prevent one HIV infection. In a study conducted by Kaiser Permanente from 2012 through 2015, 657 individuals (n = 653 MSM, n = 3 heterosexual women, and n = 1 transgender male) initiated PrEP with once daily TDF-FTC [79]. The mean age was 37 years (range 20–68 years), and mean duration of use was 7.2 months. The authors observed 388 person-years of PrEP use with no HIV diagnoses during the follow-up period. This trial adds to previous literature supporting that when taken as prescribed, PrEP is very effective. However, additional published data in women would be ideal to understand the outcomes in true clinical practice.
The big-picture relevance of PrEP will also depend on its real-world uptake. In 2015, the CDC published an analysis that estimated 492,000 MSM, 115,000 PWID, and 624,000 heterosexuals (~468,000 women) in the US alone were at substantial risk of HIV acquisition [80]. Yet, in a retrospective claims analysis of commercially insured Americans aged at least 16 years from 2010 to 2014, the number of persons prescribed TDF-FTC for PrEP was 2564 in 2014, suggesting a national estimate of 9375 persons [81]. Results stratified by gender indicated that while PrEP prevalence did increase in the female population from 1.2 per million in 2010 to 3.7 per million in 2014, both the incidence of use from year to year and the rise in prevalence were considerably lower than in the male population. Findings presented at the ASM Microbe 2016 Conference again highlighted disproportionate use among genders and a decreasing percentage of new female starts, particularly in black women [82]. These findings stress the continued need for identifying at-risk females as well as barriers to implementation of and adherence to PrEP.
PrEP uptake from a global perspective is more difficult to map but perceived as suboptimal. One of the early promising signs came from the iPrEX OLE study, an open-label extension trial of three previously enrolled PrEP trials [83]. In this study, a cohort of men and transgender women were made aware of their previous randomization assignment and then offered daily oral PrEP with TDF-FTC. Sixty-seven percent (1054 out of 1573 eligible patients) had indications for PrEP. From that group, 793 (75%) chose to use it. While the drug was provided at no cost, the study results suggested that removing barriers such as awareness, access, and provider experience led to high PrEP demand. A number of PrEP demonstration projects in Africa are planned or in progress, many of which include women. The results of these trials will be instrumental in more accurately characterizing PrEP uptake, including for special populations such as adolescent females and sex workers [84].
Conclusion
PrEP is a valuable worldwide strategy to prevent HIV infection. Unique in the sense that it requires no partner compromise or approval, PrEP may empower women who may otherwise feel burdened by social stigma or partner pressure to better protect themselves from HIV. Current evidence supports the safety and efficacy of PrEP therapy in women when taken appropriately, which is optimized by concurrent condom use and routine healthcare follow-up. However, the literature also indicates that PrEP uptake is suboptimal and increased awareness and education are needed to overcome barriers to identifying HIV risk and successfully implementing PrEP in females. Expanding options in drugs and formulations will help to encourage greater uptake, as novel agents on the horizon provide hope for increased convenience and ease of use compared to the current standard of care. Finally, widespread access, offered as part of comprehensive HIV prevention services, must be planned for and pursued. Continued research to keep up with the implementation and future changes will be required. If such efforts can prevail, the value of PrEP will increase, serving as an important pillar in the multifaceted approach to end HIV.
References
World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd edn. 2016. http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1. Accessed 28 Apr 2017.
World Health Organization. Guidance on pre-exposure oral prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: recommendations for use in the context of demonstration projects. 2012. http://www.who.int/hiv/pub/guidance_prep/en/. Accessed 25 Feb 2017.
US Public Health Service pre-exposure prophylaxis for the prevention of HIV infection in the United States—2014. A clinical practice guideline. 2014. https://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf. Accessed 25 Feb 2017.
AVAC. PrEPWatch: Country Updates. http://www.prepwatch.org/scaling-up/country-updates/. Accessed 8 Mar 2017.
UNAIDS Global summary of the AIDS epidemic. 2015. http://www.who.int/mediacentre/factsheets/fs334/en/. Accessed 8 Mar 2017.
World Health Organization Media Centre. Women’s health factsheet. 2013. http://www.who.int/mediacentre/factsheets/fs334/en/. Accessed 8 Mar 2017.
Parker RG, Easton D, Klein CH. Structural barriers and facilitators in HIV prevention: a review of international research. AIDS. 2000;14(Suppl 1):S22–32.
Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99.
Grohskopf LA, Chillag KL, Gvetadze R, Liu AY, Thompson M, Mayer KH, et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013;64(1):79–86.
Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410.
Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34.
Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22.
Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18.
Murnane PM, Celum C, Mugo N, Campbell JD, Donnell D, Bukusi E, et al. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS. 2013;27(13):2155–60.
Kahle EM, Hughes JP, Lingappa JR, John-Stewart G, Celum C, Nakku-Joloba E, et al. An empiric risk scoring tool for identifying high-risk heterosexual HIV-1 serodiscordant couples for targeted HIV-1 prevention. J Acquir Immune Defic Syndr. 2012;62:339–47.
Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90.
Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Chaipung B, et al. Factors associated with the uptake of and adherence to HIV pre-exposure prophylaxis in people who have injected drugs: an observational, open-label extension of the Bangkok Tenofovir Study. Lancet HIV. 2017;4(2):e59–66.
Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973–83.
Geary CW, Bukusi EA. Women and ARV-based HIV prevention—challenges and opportunities. J Int AIDS Soc. 2014;17(3 Suppl 2):19356.
Kwakwa HA, Bessias S, Sturgis D, Mvula N, Wahome R, Coyle C, et al. Attitudes toward HIV pre-exposure prophylaxis in a United States urban clinic population. AIDS Behav. 2016;20(7):1443–50.
Mack N, Evens EM, Tolley EE, Brelsford K, Mackenzie C, Milford C, et al. The importance of choice in the rollout of ARV-based prevention to user groups in Kenya and South Africa: a qualitative study. J Int AIDS Soc. 2014;17(3 Suppl 2):19157.
Luecke EH, Cheng H, Woeber K, Nakyanzi T, Mudekunye-Mahaka IC, van der Straten A, et al. Stated product formulation preferences for HIV pre-exposure prophylaxis among women in the VOICE-D (MTN-003D) study. J Int AIDS Soc. 2016;19(1):20875.
Corneli A, Perry B, McKenna K, Agot K, Ahmed K, Taylor J, et al. Participants’ explanations for nonadherence in the FEM-PrEP clinical trial. J Acquir Immune Defic Syndr. 2016;71(4):452–61.
Koechlin FM, Fonner VA, Dalglish SL, O’Reilly KR, Baggaley R, Grant RM, et al. Values and preferences on the use of oral pre-exposure prophylaxis (PrEP) for HIV prevention among multiple populations: a systematic review of the literature. AIDS Behav. 2017;21(5):1325–35.
Auerbach JD, Kinsky S, Brown G, Charles V. Knowledge, attitudes, and likelihood of pre-exposure prophylaxis (PrEP) use among US women at risk of acquiring HIV. AIDS Patient Care STDS. 2015;29(2):102–10.
Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):112re4.
Louissaint NA, Cao Y-J, Skipper PL, Liberman RG, Tannenbaum SR, Nimmagadda S, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retrovir. 2013;29(11):1443–50.
Dumond JB, Yeh RF, Patterson KB, Corbett AH, Jung BH, Rezk NL, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21(14):1899–907.
Seifert SM, Chen X, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA, et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. AIDS Res Hum Retrovir. 2016;32(10–11):981–91.
Cottrell ML, Yang KH, Prince HMA, Sykes C, White N, Malone S, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214(1):55–64.
Thompson CG, Cohen MS, Kashuba ADM. Antiretroviral pharmacology in mucosal tissues. J Acquir Immune Defic Syndr. 2013;63(Suppl 2):S240–7.
Mugwanya KK, Wyatt C, Celum C, Donnell D, Mugo NR, Tappero J, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175(2):246–54.
Mandala J, Nanda K, Wang M, De Baetselier I, Deese J, Lombaard J, et al. Liver and renal safety of tenofovir disoproxil fumarate in combination with emtricitabine among African women in a pre-exposure prophylaxis trial. BMC Pharmacol Toxicol. 2014;24(15):77.
Liu AY, Vittinghoff E, Sellmeyer DE, Irvin R, Mulligan K, Mayer K, et al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One. 2011;6(8):e23688.
Hoornenborh E, de Bree GJ. Acute infection with a wild-type HIV virus in PrEP use with high TDF levels. 2017 CROI Conference February 13–17, Seattle, WA, Abstract #953. http://www.croiconference.org/sessions/acute-infection-wild-type-hiv-1-virus-prep-user-high-tdf-levels. Accessed 17 May 2017.
Hanson BM, Dorais JA. Reproductive considerations in the setting of chronic viral illness. Am J Obstet Gynecol. 2017. doi:10.1016/j.ajog.2017.02.012.
Mofenson LM, Baggaley RC, Mameletzis I. Tenofovir disoproxil fumarate safety for women and their infants during pregnancy and breastfeeding. AIDS. 2017;31(2):213–32.
Benaboud S, Pruvost A, Coffie PA, Ekouévi DK, Urien S, Arrivé E, et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d’Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob Agents Chemother. 2011;55(3):1315–7.
Mugwanya KK, Hendrix CW, Mugo NR, Marzinke M, Katabira ET, Ngure K, et al. Pre-exposure prophylaxis use by breastfeeding HIV-uninfected women: a prospective short-term study of antiretroviral excretion in breast milk and infant absorption. PLoS Med. 2016;13(9):e1002132.
Abraham BK, Gulick R. Next-generation oral preexposure prophylaxis: beyond tenofovir. Curr Opin HIV AIDS. 2012;7(6):600–6.
Horn T and Jeffreys R. Preventative technologies: antiretroviral and vaccine development. http://www.pipelinereport.org/sites/default/files/201607/HIV%20Prevention.pdf. Accessed 14 Apr 2017.
Massud I, Mitchell J, Babusis D, Deyounks F, Ray AS, Rooney JF, et al. Chemoprophylaxis with oral emtricitabine and tenofovir alafenamide combination protects macaques from rectal simian/human immunodeficiency virus infection. J Infect Dis. 2016;214(7):1058–62.
Gunawardana M, Remedios-Chan M, Miller CS, Fanter R, Yang F, Marzinke MA, et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob Agents Chemother. 2015;59(7):3913–9.
Schlesinger E, Johengen D, Luecke E, et al. A tubable, biodegradable, thin-film polymer device as a long-acting implant delivering tenofovir alafenamide fumarate for HIV pre-exposure prophylaxis. Pharm Res. 2016;33(7):1649–56.
Garrett KL, Cottrell ML, Prince HM, Sykes C, Schauer A, Peery A, et al. Concentrations of TFV and TFVdp in female mucosal tissues after a single dose of TAF. 2016 CROI Conference February 22–25, Boston, MA, Poster #102LB. http://www.croiconference.org/sessions/concentrations-tfv-and-tfvdp-female-mucosal-tissues-after-single-dose-taf. Accessed 3 Apr 2017.
National Institutes of Health Clinicaltrials.gov. Safety and efficacy of emtricitabine and tenofovir alafenamide (F/TAF) fixed-dose combination once daily for pre-exposure prophylaxis in men and transgender women who have sex with men and are at risk for HIV-1 infection (DISCOVER). https://clinicaltrials.gov/ct2/show/NCT02842086. Accessed 20 May 2017.
Ohrui H, Kohgo S, Hayakawa H, Kodama E, Matsuoka M, Nakata T, Mitsuya H. 2′-deoxy-4′-C-ethynyl-2-fluoroadenosine: a nucleoside reverse transcriptase inhibitor with highly potent activity against wide spectrum of HIV-1 strains, favorable toxic profiles, and stability in plasma. Nucleos Nucleot Nucl. 2007;26:1543–6.
Kovarova M, Shanmugasundaram U, Baker CE, et al. HIV pre-exposure prophylaxis for women and infants prevents vaginal and oral HIV transmission in a preclinical model of HIV infection. J Antimicrob Chemother. 2016;71:3185–94.
Dumond JB, Patterson KB, Pecha AL, Werner RE, Andrews E, Damle B, et al. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009;51(5):546–53.
Neff CP, Ndolo T, Tandon A, Habu Y, Akkina R. Oral pre-exposure prophylaxis by anti-retrovirals raltegravir and maraviroc protects against HIV-1 vaginal transmission in a humanized mouse model. PLoS One. 2010;5(12):e15257.
Massud I, Aung W, Martin A, Bachman S, Mitchell J, Aubert R, et al. Lack of prophylactic efficacy of oral maraviroc in macaques despite high drug concentrations in rectal tissues. J Virol. 2013;87(16):8952–61.
Coll J, Moltó J, Boix J, Gómez-Mora E, Else L, García E, Paredes R, Ouchi D, Carillo A, Escrig R, Back D, Clotet B, Cabrera C. Single oral dose of maraviroc does not prevent ex vivo HIV infection of rectal mucosa in HIV-1 negative human volunteers. AIDS. 2015;29(16):2149–54.
Moss JA, Butkyavichene I, Churchman SA, et al. Combination pod-intravaginal ring delivers antiretroviral agents for HIV prophylaxis: pharmacokinetic evaluation in an ovine model. Antimicrob Agents Chemother. 2016;60(6):3759–66.
Gulick RM, Wilkin TJ, Chen YQ, et al. Phase 2 Study of the safety and tolerability of maraviroc-containing regimens to prevent HIV infection in men who have sex with men (HPTN 069/ACTG A5305). J Infect Dis. 2017;215(2):238–46.
Gulick RM et al. HPTN 069/ACTG A5305: maraviroc-containing PrEP regimens safe, tolerable in cohort of US women. 21st International AIDS Conference 2016, Durban, South Africa. http://www.natap.org/2016/IAC/IAC_15.htm. Accessed 14 Apr 2017.
Next PrEP. HPTN 069/ACTG 5305. http://www.nextprepstudy.org/about.html. Accessed 6 Apr 2017.
Jackson AGA, Else LJ, Mesquita PMM, Egan D, Back DJ, Karolia Z, et al. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin Pharmacol Ther. 2014;96(3):314–23.
Verloes R, Deleu S, Niemeijer N, Crauwels H, Meyvisch P, Williams P. Safety, tolerability and pharmacokinetics of rilpivirine following administration of a long-acting formulation in healthy volunteers. HIV Med. 2015;16(8):477–84.
McGowan I, Dezzutti CS, Siegel A, Engstrom J, Nikiforov A, Duffill K, et al. Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): an open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV. 2016;3(12):e569–78.
Bekker LG, Li S, Tolley E, et al. HPTN 076: TMC278 LA safe, tolerable and acceptable for HIV pre-exposure prophylaxis. 2017 CROI Conference February 13-17, Seattle, WA, Poster #2429. http://www.croiconference.org/sessions/hptn-076-tmc278-la-safe-tolerable-and-acceptable-hiv-preexposure-prophylaxis. Accessed 14 Apr 2017.
National Institutes of Health Clinicaltrials.gov. Phase II safety and acceptability of an investigation injectable product, TMC278LA, for pre-exposure prophlyaxis (TMC278LA). https://clinicaltrials.gov/ct2/show/NCT02165202 (Identification No. NCT02165202). Accessed 14 Apr 2017.
McGowan I. The promise and challenges of sustained delivery of PrEP. 2016 CROI Conference February 22–25, Boston, MA, Abstract #71. http://www.croiconference.org/sessions/promise-and-challenges-sustained-delivery-prep. Accessed 14 Apr 2017.
das Neves J, Martins JP, Sarmento B. Will dapivirine redeem the promises of anti-HIV microbicides? Overview of product design and clinical testing. Adv Drug Deliv Rev. 2016;103:20–32.
Rees H, Delany-Moretlwe SA, Lombard C, Baron D, Panchia R, Myer L, et al. FACTS 001 phase III trial of pericoital tenofovir 1% gel for preventionin women. 2015 CROI Conference February 23–26, Seattle, WA, Abstract #26LB. http://www.croiconference.org/sessions/facts-001-phase-iii-trial-pericoital-tenofovir-1-gel-hiv-prevention-women. Accessed 14 Apr 2017.
International Partnerships for Microbicides. Sister studies: the ring study and ASPIRE. February 2016. http://www.ipmglobal.org/publications/sister-studies-ring-study-and-aspire. Accessed 2 Apr 2017.
Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375(22):2121–32.
Nel A, van Niekerk N, Kapiga S, Bekker L-G, Gama C, Gill K, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375(22):2133–43.
Balkus J, Palanee-Phillips T, Siva S, Nakabiito C, Nair G, Kabwigu S, et al. Dapivirine ring use does not diminish the effectiveness of hormonal contraception. 2017 CROI Conference February 13–17, Seattle, WA, Abstract #88. http://www.croiconference.org/sessions/dapivirine-ring-use-does-not-diminish-effectiveness-hormonal-contraception. Accessed 14 Apr 2017.
Makanani B, Balkus J, Palanee-Phillips T, Mbilizi Y, Piper J, Kintu K, et al. Pregnancy incidence and outcome samong women using the dapivirine vaginal ring. 2017 CROI Conference February 13-17, Seattle, WA, Abstract #935. http://www.croiconference.org/sessions/pregnancy-incidence-and-outcomes-among-women-using-dapivirine-vaginal-ring. Accessed 14 Apr 2017.
Giacobbi NS, Sluis-Cremer. Cross-resistance profiles of the NNRTIs in development to prevent HIV-1 infection. 2017 CROI Conference February 13-17, Seattle, WA, Abstract #501. http://www.croiconference.org/sessions/cross-resistance-profiles-nnrtis-development-prevent-hiv-1-infection. Accessed 14 Apr 2017.
Whitfield T, Torkington A, van Halsema C. Profile of cabotegravir and its potential in the treatment and prevention of HIV-1 infection: evidence to date. HIV AIDS (Auckl). 2016;8:157–64.
Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014;67(5):481–6.
Spreen W, Williams P, Margolis D, Ford SL, Crauwels H, Lou Y, et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr. 2014;67(5):487–92.
HIV Prevention Trials Network. HPTN 077: A phase IIa study to evaluate the safety, tolerability and pharmacokinetics of the investigational injectable HIV integrase inhibitor, GSK1265744, in HIV-uninfected men and women. https://www.hptn.org/sites/default/files/2016-05/077V1_01May14.pdf. Accessed 6 Apr 2017.
National Institutes of Health Clinicaltrials.gov. Safety and efficacy study of injectable cabotegravir compared to daily oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), for pre-exposure prophylaxis in HIV-uninfected cisgender men and transgender women who have sex with men. from: https://clinicaltrials.gov/show/NCT02720094. Accessed 14 Apr 2017.
Margolis DA, Brinson CC, Smith GHR, de Vente J, Hagins DP, Eron JJ, et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15(10):1145–55.
Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23):2237–46.
McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomized trial. Lancet. 2016;387(10013):53–60.
Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, FOllansbee S, Hare CB. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61:1601–3.
Smith DK, Van Handel M, Wolitski RJ, Stryker JE, Hall HI, Prejean J, et al. Vital signs: estimated percentages and numbers of adults with indications for preexposure prophylaxis to prevent HIV acquisition—United States, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(46):1291–5.
Wu H, Mendoza MCB, Huang Y-LA, Hayes T, Smith DK, Hoover KW. Uptake of HIV preexposure prophylaxis among commercially insured persons-United States, 2010-2014. Clin Infect Dis. 2017;64(2):144–9.
Bush S, Magnuson D, Rawlings MK, Hawkins T, McCallister S, Giler M, et al. Racial Characteristics of FTC/TDF for Pre-exposure Prophylaxis (PrEP) Users in the US. 2016 ASM Microbe/ICAAC, Boston, MA. http://www.natap.org/2016/HIV/062216_02.htm. Accessed 14 Apr 2017.
Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–9.
Cowan FM, Delany-Moretlwe S, Sanders EJ, Mugo NR, Guedou FA, Alary M, et al. PrEP implementation research in Africa: what is new? J Int AIDS Soc. 2016;19(7(Suppl 6)):21101.
European AIDS Clinical Society. Guidelines Version 8.2. http://www.eacsociety.org/files/guidelines_8.2-english.pdf. Published January 2017. Accessed 17 May 2017.
Hattori S, Ide K, Nakata H, Harada H, Suzu S, Ashida N, et al. Potent activity of a nucleoside reverse transcriptase inhibitor, 4′-ethynyl-2-fluoro-2′-deoxyadenosine, against human immunodeficiency virus type 1 infection in a model using human peripheral blood mononuclear cell-transplanted NOD/SCID Janus kinase 3 knockout mice. Antimicrob Agents Chemother. 2009;53(9):3887–93.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Jennifer L. Bailey, Suzanne T. Molino, Ana D. Vega, and Melissa Badowski all declare there are no disclosures related to personal, financial, commercial, or academic conflicts of interests.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/D598F06079036738.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bailey, J.L., Molino, S.T., Vega, A.D. et al. A Review of HIV Pre-Exposure Prophylaxis: The Female Perspective. Infect Dis Ther 6, 363–382 (2017). https://doi.org/10.1007/s40121-017-0159-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-017-0159-9