Abstract
Introduction
Ceftolozane/tazobactam (C/T) is a novel antibiotic approved for complicated intra-abdominal and urinary tract infections caused by Gram-positive and Gram-negative organisms, including some MDR strains. Little is known about the use of this agent for treatment of bacteremia and even less so about the appropriateness of the renally defined regimens. We describe a case of a 66-year-old man with a history of chronic kidney disease (baseline Cr = 3–4 mg/dl) and recurrent nephrolithiasis with bilateral stents who had positive concurrent urine and blood cultures for MDR Pseudomonas aeruginosa (PSA), susceptible only to amikacin and colistin. Due to the MDR phenotype and his underlying kidney disease, the 375 mg (250 mg/125 mg) dose of C/T was given as monotherapy every 8 h for his bloodstream infection.
Methods
Once steady state was anticipated, blood was obtained at the end of infusion (1 h), and at 3, 5 and 8 h for drug concentration determination using a validated high-performance liquid chromatography method at the Center for Anti-Infective Research and Development, Hartford Hospital, Hartford.
Results
The minimum inhibitory concentration (MIC) for the PSA was 2/4 for C/T, indicating susceptibility. Concentration of ceftolozane of 21.87 µg/ml at 8 h indicated that serum concentrations were maintained above the MIC throughout the dosing interval. The patient was given 25 days of C/T and experienced a successful clinical outcome. Blood cultures obtained at 1 and 3 weeks after completion of treatment remained sterile. No adverse events were attributed to C/T.
Conclusion
In this patient, the renally adjusted dose of C/T was safe and provided sufficiently high drug concentrations that exceeded the MIC of the infecting organism over the course of therapy. More data are required to determine the clinical utility of C/T in the setting of MDR PSA bacteremia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ceftolozane/tazobactam (C/T) is a novel beta-lactam/beta-lactamase inhibitor combination antibiotic, recently approved in December 2014. It offers broad spectrum coverage for many Gram-positive and Gram-negative organisms, including some multi-drug-resistant (MDR) strains [1]. In addition to activity against some AmpC beta-lactamases and extended-spectrum b-lactamase-producing Enterobacteriaceae, C/T has demonstrated impressive antipseudomonal in vitro activity. It has been shown recently in a large US surveillance study to be among the most potent antipseudomonal agents currently available, retaining activity against cefepime, piperacillin/tazobactam and carbapenem non-susceptible isolates as well as those defined as MDR [2].
C/T is currently approved by the United States Food and Drug Administration (FDA) for treatment of complicated intra-abdominal infections (cIAI), in combination with metronidazole, as well as for complicated urinary tract infections (cUTI), including pyelonephritis [1, 3]. Ongoing Phase 3 trials are currently in progress for the treatment of ventilator-associated and nosocomial pneumonia [4]. While patients with concurrent bacteremia were included in Phase 3 studies, there were few, and thus little is known about the use of this agent for treatment of bacteremia, specifically due to Pseudomonas aeruginosa (PSA) and the appropriateness of the recommended renally adjusted regimens [5, 6]. We describe a case of a 66-year-old man with a history of chronic kidney disease and recurrent nephrolithiasis with bilateral stents who had positive concurrent urine and blood cultures for MDR PSA, susceptible only to amikacin and colistin. Due to the MDR phenotype and his underlying kidney disease, a renally adjusted C/T monotherapy regimen was initiated for his bloodstream infection (BSI).
Case Presentation
A 66-year-old Caucasian man with past medical history significant for morbid obesity, chronic kidney disease (baseline Cr 3.0–3.4 mg/dl; estimated creatinine clearance CrCl ~35 ml/min; not on dialysis), right-sided heart failure, hypothyroidism, recurrent nephrolithiasis and urinary tract infections with multiple urologic procedures, was admitted to the hospital with complaints of fevers and chills. Blood cultures taken on admission returned positive for Proteus mirabilis. Initial urine cultures revealed P. mirabilis and Klebsiella pneumoniae and a repeat urine culture a day later revealed P. mirabilis along with an MDR PSA. CT preformed of the abdomen showed an obstructing 7 mm calculus within the proximal right ureter, near the right ureteropelvic junction. Right hydronephrosis and additional calculi within the right renal pelvis were also noted. The patient was treated with ceftriaxone and underwent cystoscopy, bilateral retrograde pyelogram and bilateral stent placement the day after admission. The patient was transferred to the long-term care center of the hospital where he completed 2 weeks of ceftriaxone for the bacteremia and then was continued on the same antibiotic as suppressive therapy until another upcoming genitourinary procedure, given the patient’s history of recurrent infections.
Approximately 3 weeks after this initial episode of Proteus bacteremia, the patient developed fevers and chills, and urine and blood cultures both revealed MDR PSA, susceptible only to amikacin and colistin. The patient had initially been placed on piperacillin/tazobactam as empiric therapy, but when susceptibility results returned, a switch was made to amikacin while awaiting susceptibility results for C/T which had been requested. He was continued on amikacin for a week during which time he underwent a cystoscopy, bilateral ureteroscopy and bilateral stent exchange. Post-procedure, the patient developed shaking chills and fever. Blood cultures were obtained and again revealed MDR PSA, this time sensitive only to colistin. C/T susceptibility results from the previously positive blood culture had returned by this time. The minimum inhibitory concentration (MIC) for the PSA was 2/4 µg/ml for C/T, indicating susceptibility (susceptible = MIC ≤4/4 µg/ml based on FDA susceptibility criteria) [1]. Amikacin was discontinued and monotherapy with C/T at the renally adjusted dose of 375 mg (250 mg/125 mg) was initiated intravenously every 8 h, according to manufacturer recommendations [1]. Colistin was avoided due to the patient’s worsening renal function in the setting of his CKD. The second MDR PSA isolate from the later bacteremia had also tested susceptible to C/T, with the same MIC. The patient tolerated this therapy well, with prompt resolution of symptoms and clearance of bacteremia. The first negative blood culture was drawn approximately an hour after first dose of C/T was given. Two weeks after the stents had been exchanged, they were removed. Blood cultures taken the same day as the stent removal were negative. The planned duration of C/T therapy was 2 weeks post-stent exchange, with the patient receiving a total of 25 days of therapy. Blood cultures obtained at 1 week, 11 days, 3 weeks and 7 weeks after completion of C/T all remained sterile.
Methods and Results
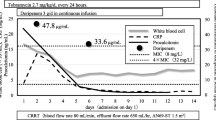
The patient had received 46 doses of C/T (well after the fifth dose when steady state is expected to be achieved) before serum drug levels were checked. Blood was obtained at the end of infusion (1 h) and at 3, 5 and 8 h for drug concentration determination using a validated high-performance liquid chromatography method at the Center for Anti-Infective Research and Development, Hartford Hospital, Hartford [7]. Total C/T serum concentrations at 1, 3, 5 and 8 after the start of the infusion were 41.4/6.9, 36.8/5.1, 28.7/3.2 and 21.9/2.8 µg/ml, respectively. Using a non-compartmental pharmacokinetic approach, the area under the concentration–time curve (AUC) over the dosing interval was calculated to be 473 µg h/ml for C, while its half-life was estimated to be 7.4 h. Importantly, the recommended renal adjusted dose produced concentrations of C that were maintained above the MIC of the infecting PSA as well as the susceptibility breakpoint of 4 µg/ml through the entire dosing interval. As a result of the low protein binding of C, approximately 19% [1], even if free drug concentrations were to be estimated, concentrations would still be expected to be well above the 4 µg/ml breakpoint value. The patient did not experience any adverse effects attributed to C/T. Informed consent was obtained from all patients for being included in the study.
Discussion
C/T demonstrated efficacy against an MDR PSA bacteremia from a presumed genitourinary source in this patient. It was found to be safe and provided sufficiently high drug concentrations that exceeded the MIC of the infecting organism over the course of therapy. In an era where few novel antimicrobial agents are available to combat the increasing trend in MDRs, newer antibiotics such as C/T are welcome additions to the limited treatment options. Although not currently indicated for bacteremia, C/T demonstrates excellent in vitro activity against MDR PSA and possesses appealing potential for these infections.
While Phase 3 studies have demonstrated clinical and microbiological efficacy of C/T for PSA isolates, very few subjects had concurrent bacteremia. In the ASPECT-cUTI trial (ClinicalTrials.gov identifier, NCT01345929) where C/T was compared with levofloxacin in the treatment of UTIs, including pyelonephritis, 7.3% versus 8.2% had associated bacteremia in the C/T and levofloxacin groups, respectively [5]. The main etiology for bacteremia was Escherichia coli and occurred in patients with pyelonephritis. C/T had a composite cure of 79.3% in patients with bacteremia vs. 57.6% for levofloxacin; however, the breakdown of etiologic organisms per specific culture site was not disclosed in the study. Although C/T resulted in microbiological eradication in 85.7% (6/7) of subjects with PSA as the baseline pathogen, the incidence was small and thus statistical conclusions could not be drawn [5].
Similarly, in the ASPECT-cIAI (ClinicalTrials.gov identifier, NCT01445678) trial where C/T in addition to metronidazole was compared to meropenem for treatment of cIAI, PSA accounted for 8.9% of baseline pathogens from intra-abdominal specimens, with the majority of infections being polymicrobial [6]. MDR PSA isolates, though present in this study, accounted for 11.5% of all individual PSA isolates. Susceptibility rates to C/T and meropenem for PSA were 98.6% and 89.9%, respectively. Clinical cure of PSA infection was demonstrated in 100% (26/26) of infections treated with C/T plus metronidazole and in 93.1% (27/29) treated with meropenem. Concurrent bacteremia was only present in about 2% of patients in each treatment arm. Interestingly, in sub-group analyses of the microbiologically evaluable population, lower clinical cure rates were seen in patients with moderate renal impairment (CrCl <50 ml/min), in both treatment groups. Patients with severe renal impairment (CrCl <30 ml/min) were excluded from the study [6]. Our patient falls into this latter category, and while there was no guidance as to effective dosing from the literature, manufacturer recommendations were used to guide dosing [1]. Importantly, the pharmacokinetic data derived from this case confirm the adequacy of the manufacturers’ defined renal dose in a bacteremic patient with multiple co-morbid conditions as the concentrations were maintained well above the susceptibility breakpoint value of 4 µg/ml. Moreover, the overall exposure as defined by the AUC for this dosing regimen was quite similar to dose-adjusted exposures previously defined in non-infected renal patients [8].
Rates of infection due to MDR PSA are increasing, leading to increased mortality and healthcare costs in addition to treatment challenges for clinicians [9–11]. Often, very few therapeutic options exist and patient allergies and intolerance to antimicrobials can limit these options even further. Emergence of resistance occurring during treatment of initially susceptible infections also complicates management of these infections. Consideration should be given to the pharmacokinetics and pharmacodynamics of an antimicrobial agent to optimize dosing, particularly in the treatment of MDR infections, as subtherapeutic drug levels can lead to development of resistance. Activity of beta-lactam antibiotics is determined by the proportion of the dosing interval that the free drug remains above the MIC (T > MIC). Specifically for cephalosporins, the goal T > MIC is considered 60–70% [12]. The challenge arises when treating organisms associated with higher MICs, such as PSA, as target drug levels are often unattainable when using conventional dosing of older antibiotics. In an attempt to optimize exposures, alternative dosing methods such as extended and continuous infusions of beta-lactams aim to extend the T > MIC, thus theoretically increasing efficacy and offering a potential benefit when treating infections associated with higher MICs.
While the above dosing interventions may be required to optimize the exposures of less potent compounds, the utilization of the C/T renally adjusted regimen in the current case offered the opportunity to provide exposures (i.e., AUC and T > MIC) similar to that expected after the administration of the 2000/1000 mg regimen in patients with normal renal function. While this regimen provided an optimized pharmacodynamic profile which resulted in a rapid clinical response and eradication of this MDR pathogen, the exposure obtained in this patient was also similar to that observed by VanScoy and colleagues as being required to prevent the amplification of PSA-drug-resistant bacterial subpopulations using a hollow-fiber infection model [13].
Though the controversy of combination therapy versus monotherapy has not fully been elucidated, therapy for severe infections with two antipseudomonal agents are often empirically started to avoid inadequate therapy or treatment failure [14]. Multi-drug resistance has been shown to be associated with inappropriate empirical antibiotic therapy, and although inappropriate initial coverage for PSA BSI has been shown to be an independent risk factor for mortality, there is some conflicting evidence in the literature [15–19]. Successful regimens for MDR PSA infections, mainly described in the form of case reports and retrospective reviews, have included continuous-infusion meropenem, parenteral colistin therapy with and without the addition of rifampin, and combination therapy with antipseudomonal beta-lactams plus aminoglycosides [15, 16, 20]. Double antipseudomonal beta-lactam therapy with or without the addition of an aminoglycoside has been shown in vitro to have synergy effects despite resistance to one or more of these agents, though clinical data are lacking [21, 22].
In vitro studies have demonstrated C/T to have high activity against pseudomonal isolates, including MDR strains [2]. In addition to the recent study by Sutherland and Nicolau, Farrell DJ, et al. described the activity of C/T against 7071 Enterobacteriaceae and 1971 PSA isolates collected from US hospitals between January 2011 and December 2012 [23]. Among all antipseudomonal antibiotics tested, including ceftazidime, meropenem, piperacillin/tazobactam, levofloxacin, gentamicin, and colistin, C/T was the most potent agent tested, inhibiting 96.1% of isolates, at an MIC of ≤4 µg/ml. C/T was the second most active agent, after colistin, against 310 MDR PSA isolates, defined as non-susceptibility to ≥1 agent in ≥3 antimicrobial classes. In this analysis, there was only one isolate that showed resistance to all antimicrobial classes, and C/T demonstrated no observable activity against it. C/T was, however, shown to maintain activity in strains that showed combined resistance to multiple antibiotics, including ceftazidime, meropenem, and piperacillin/tazobactam.
Conclusion
The package insert recommended renal dose of C/T was used successfully and safely as monotherapy to treat an MDR PSA bacteremia in a patient with a presumed genitourinary source and severely compromised renal function. More data are required to determine the clinical utility of C/T in the setting of MDR PSA bacteremia as well as various degrees of renal dysfunction.
References
Cubist Pharmaceuticals US Zerbaxa (ceftolozane/tazobactam) package insert. Lexington, MA; 2014.
Sutherland CA, Nicolau DP. Susceptibility profile of Ceftolozane/tazobactam and other parenteral antimicrobials against Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa from US Hospitals. Clin Ther. 2015;37(7):1564–71.
Cho JC, Fiorenza MA, Estrada SJ. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination. Pharmacotherapy. 2015;35(7):701–15.
US National Institutes of Health. ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT02070757?term=ceftolozane+tazobactam&rank=3. Accessed October 28, 2015.
Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet. 2015;385:1949–56.
Solomkin J, Hershberger E, Miller B, et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis. 2015;60(10):1462–71.
Sutherland CA, Nicolau DP. Development of an HPLC Method for the determination of ceftolozane/tazobactam in biological and aqueous matrixes. J Chromatogr Sci (in press).
Wooley M, Miller B, Krishna G, Hershberger E, Chandorkar G. Impact of renal function on the pharmacokinetics and safety of ceftolozane–tazobactam. Antimicrob Agents Chemother. 2014;58(4):2249–55.
Nathwani D, Raman G, Sulham K, Gavaghan M, Menon V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2014;3(1):32.
Karlowsky JA, Draghi DC, Jones ME, Thornsberry C, Friedland IR, Sahm DF. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob Agents Chemother. 2003;47:1681–8.
Obritsch MD, Fish DN, MacLaren R, Jung R. National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob Agents Chemother. 2004;48:4606–10.
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–12.
VanScoy BD, Mendes RE, Castanheira M. Relationship between ceftolozane-tazobactam exposure and selection for Pseudomonas aeruginosa resistance in a hollow-fiber infection model. Antimicrob Agents Chemother. 2014;58(10):6024–31.
Traugott KA, Echevarria K, Maxwell P, Green K, Lewis JS 2nd. Monotherapy or combination therapy? The Pseudomonas aeruginosa conundrum. Pharmacotherapy. 2011;31(6):598–608.
McCarthy K. Pseudomonas aeruginosa: evolution of antimicrobial resistance and implications for therapy. Semin Respir Crit Care Med. 2015;36:44–55.
Kang CI, Kim SH, Kim HB, et al. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis. 2003;37(6):745–51.
Morata L, Cobos-Trigueros N, Martínez JA, et al. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2012;56(9):4833–7.
Lodise TP, Patel N, Kwa A, et al. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother. 2007;51(10):3510–5.
Osih RB, McGregor JC, Rich SE, et al. Impact of empiric antibiotic therapy on outcomes in patients with Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2007;51(3):839–44.
Obritsch MD, Fish DN, MacLaren R, Jung R. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 2005;25(10):1353–64.
Oie S, Sawa A, Kamiya A, Mizuno H. In-vitro effects of a combination of antipseudomonal antibiotics against multi-drug resistant Pseudomonas aeruginosa. J Antimicrob Chemother. 1999;44:689–91.
Oie S, Uematsu T, Sawa A, et al. In vitro effects of combinations of antipseudomonal agents against seven strains of multidrug-resistant Pseudomonas aeruginosa. J Antimicrob Chemother. 2003;52:911–4.
Farrell DJ, Flamm RK, Sader HS, Jones RN. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in US Hospitals (2011–2012). Antimicrob Agents Chemother. 2013;57:6305–10.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Ursula Patel, David Nicolau, and Rabeeya Sabzwari have nothing to disclose.
Compliance with Ethics Guidelines
Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content To view enhanced content for this article go to http://www.medengine.com/Redeem/5844F060773732EA.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Patel, U.C., Nicolau, D.P. & Sabzwari, R.K. Successful Treatment of Multi-Drug Resistant Pseudomonas aeruginosa Bacteremia with the Recommended Renally Adjusted Ceftolozane/Tazobactam Regimen. Infect Dis Ther 5, 73–79 (2016). https://doi.org/10.1007/s40121-016-0104-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-016-0104-3