Abstract
Introduction
This study assessed the cost-effectiveness of ozanimod compared with commonly used disease-modifying therapies (DMTs) for relapsing–remitting multiple sclerosis (RRMS).
Methods
Annualized relapse rate (ARR) and safety data were obtained from a network meta-analysis (NMA) of clinical trials of RRMS treatments including ozanimod, fingolimod, dimethyl fumarate, teriflunomide, interferon beta-1a, interferon beta-1b, and glatiramer acetate. ARR-related number needed to treat (NNT) relative to placebo and annual total MS-related healthcare costs was used to estimate the incremental annual cost per relapse avoided with ozanimod vs each DMT. ARR and adverse event (AE) data were combined with drug costs and healthcare costs to manage relapses and AEs in order to estimate annual cost savings with ozanimod vs other DMTs, assuming a 1 million USD fixed treatment budget.
Results
Treatment with ozanimod was associated with lower incremental annual healthcare costs to avoid a relapse, ranging from $843,684 vs interferon beta-1a (30 μg; 95% confidence interval [CI] − $1,431,619, − $255,749) to $72,847 (95% CI − $153,444, $7750) vs fingolimod. Compared with all other DMTs, ozanimod was associated with overall healthcare cost savings ranging from $8257 vs interferon beta-1a (30 μg) to $2178 vs fingolimod. Compared with oral DMTs, ozanimod was associated with annual cost savings of $6199 with teriflunomide 7 mg, $4737 with teriflunomide 14 mg, $2178 with fingolimod, and $2793 with dimethyl fumarate.
Conclusion
Treatment with ozanimod was associated with substantial reductions in annual drug costs and total MS-related healthcare costs to avoid relapses compared with other DMTs. In the fixed-budget analysis, ozanimod demonstrated a favorable cost-effective profile relative to other DMTs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This analysis provides healthcare stakeholders with insights into the cost-effectiveness associated with the use of ozanimod versus other self-administered disease-modifying therapies (DMTs) for relapsing-remitting multiple sclerosis (RRMS). |
Treatment with ozanimod was associated with substantial reductions in annual drug costs and total MS-related healthcare costs to avoid relapses compared with all other DMTs included in the analysis; these reductions were significant versus glatiramer acetate (20 mg), teriflunomide, interferon beta-1b, and interferon beta-1a. |
Treatment with ozanimod was associated with cost savings compared with the other DMTs included in the analysis. |
Introduction
Relapsing–remitting multiple sclerosis (RRMS) is an immune-mediated, neurodegenerative disease characterized by episodes of relapses followed by periods of remission [1]. Although episodes of neurologic dysfunction are often followed by periods of recovery, relapses can be associated with sustained disability [2,3,4]. Over time, most patients transition into the secondary progressive phase [5], which has a significant impact on patients’ quality of life and imposes a higher economic burden [6]. Among patients with RRMS, the occurrence of relapses in a given year is also associated with higher healthcare costs [5, 7]. The overall economic burden of multiple sclerosis (MS) is very high. A recent US study [8] estimated the total economic burden to be $85.4 billion (direct medical costs of $63.3 billion and indirect and nonmedical costs of $22.1 billion), and the excess per capita annual medical costs of patients with MS was $65,612 more than for individuals without MS.
Since the approval of the first disease-modifying therapy (DMT) for MS nearly 30 years ago, the treatment landscape has rapidly expanded [9, 10], and there are now over a dozen DMTs approved by the US Food and Drug Administration (FDA) [11]. DMTs are effective in reducing relapse rates and delaying disease progression, which in addition to preserving function and quality of life can also lead to savings in the overall cost of care. However, MS requires lifelong management and the cost of treatment with existing DMTs remains high [12]. Moreover, differences in dosing, administration, and adverse events (AEs) are important factors affecting patients’ tolerance of and adherence to these therapies [13].
Ozanimod is a relatively new oral DMT that received FDA approval in 2020 [14]. Taken orally once daily, ozanimod is a sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5, inhibiting immune cell migration to the central nervous system [14]. In a recently published network meta-analysis (NMA) [15], the annualized relapse rate (ARR) with ozanimod was significantly lower than the rate with other first-line oral and injectable DMTs for RRMS (interferon beta-1a [22, 30, and 44 μg], interferon beta-1b [250 μg], glatiramer acetate 40 mg, and teriflunomide [7 mg and 14 mg]) and similar to the rate with dimethyl fumarate (240 mg) and fingolimod 0.5 mg. The rates of any AE and serious AEs (SAEs) with ozanimod were also comparable to those with other oral DMTs. In a subsequent number needed to treat (NNT) analysis, ozanimod demonstrated an improved relapse rate and a better safety profile than placebo and other DMTs [16].
Given that available DMTs vary in their ability to reduce relapse and AE rates, as well as in their annual cost per patient, it is important to jointly evaluate the health and economic impacts of DMTs commonly used for the treatment of patients with RRMS. However, other published cost-effectiveness analyses of DMTs in RRMS have not included ozanimod among the comparators [17,18,19]. The purpose of this study, therefore, was to assess the cost-effectiveness of ozanimod compared with other oral and injectable DMTs (i.e., self-administered therapies) using several measures, including incremental drug costs per relapse avoided, MS-related healthcare costs per relapse avoided, and cost savings associated with avoiding relapses and AEs with ozanimod vs comparator DMTs over a 1-year period, with a goal of providing healthcare decision makers in the USA with a more complete and current assessment of the RRMS treatment landscape.
Methods
Data Sources
Comparative data were obtained from previously conducted NMA [15] and NNT [16] analyses of ARR and AEs for DMTs. Analyses included commonly used first-line oral DMTs (ozanimod [1 mg], fingolimod [0.5 mg], dimethyl fumarate [240 mg], and teriflunomide [7 and 14 mg]) and injectable DMTs (interferon beta-1a [22, 30, and 40 μg], interferon beta-1b [250 μg], and glatiramer acetate) for the treatment of RRMS. Notably, the NMA networks did not include AE data for interferon beta-1a (22 μg) and interferon beta-1b (250 μg); these treatments were therefore excluded from any analyses that relied on these comparative safety data.
Data on annual costs of treatment with selected DMTs were obtained from the Red Book (IBM 2020) online drug pricing database [20]. Healthcare cost data associated with relapses were obtained from a targeted literature review. Healthcare cost data associated with managing AEs and SAEs were obtained from the Healthcare Cost and Utilization Project data set and the Centers for Medicare and Medicaid Services database [21].
Compliance with Ethics Guidelines
No new data were collected in the course of this study, and approval from an ethics committee was not required to conduct this analysis. The data sets used are publicly available and did not require permission to use. The study does not involve human subjects; all studies included in the model we report conformed with the Helsinki Declaration of 1964 and its amendments.
Analyses
Annual Drug Costs
Drug costs (in 2020 US dollars) were calculated on the basis of dosing regimens approved by the FDA for RRMS. Wholesale acquisition costs (WAC) per unit dose were obtained from the Red Book Online database [20]. Using these data, we calculated annual drug costs per person for each DMT of interest by multiplying the drug WAC per unit dose by the total dose required over a 1-year period (Online Resource 1 in the electronic supplementary material).
Incremental Annual Drug Costs to Avoid a Relapse
The cost per relapse avoided for each DMT over a 1-year period was calculated as the product of the NNT [16] to avoid a relapse and annual drug costs per person. Incremental annual drug costs per relapse avoided for ozanimod vs each comparator drug were calculated as the difference in annual drug costs to avoid a relapse between ozanimod and each comparator. A negative value for incremental annual drug costs indicated that the drug costs to avoid one relapse were lower for ozanimod than for the comparator. The 95% CI for the incremental drug costs was calculated using the delta method, assuming normality and independence of the rate/risk difference outcome data obtained from the NMA [15].
Cost of Managing a Relapse
Healthcare cost data associated with managing relapses in RRMS were extracted from selected references identified by a targeted literature review. Cost data were transformed to 2020 US dollars [22]. Average healthcare cost estimates in the identified references were used for the analysis [23,24,25,26]. Online Resource 2 in the electronic supplementary material provides a summary of relapse-related costs. Sensitivity analyses were conducted to assess the impact of changing this cost estimate on the outcomes of interest.
Cost of Managing an Adverse Event
There were a large number of possible AEs to consider in order to estimate the average cost of managing an AE in patients with RRMS. To simplify this calculation, we first estimated the cost of managing an SAE and nonserious AE and used these values to estimate the cost of managing an AE (described in detail in Online Resources 3 and 4 in the electronic supplementary material). The cost of managing any AE in a patient with RRMS was calculated as follows:
where w is the relative frequency of SAEs vs nonserious AEs and was determined from safety data reported in the ozanimod RADIANCE trial [27] (Online Resource 5 in the electronic supplementary material). Sensitivity analyses were also conducted to assess the impact of changing this cost estimate on outcomes.
Total Annual MS-Related Healthcare Costs and Total Costs Per Relapse Avoided
The total annual healthcare cost per relapse avoided was calculated as follows:
The probability of an AE per treatment per year was obtained from the treatment arm-level NMA results (the probability of relapse was derived from ARR NMA data). The total healthcare cost per relapse avoided for each treatment was calculated as follows:
Thus, the incremental total annual MS-related healthcare cost per relapse avoided for ozanimod vs each comparator was calculated as the difference between corresponding MS-related healthcare cost per relapse avoided. A negative value for incremental MS-related healthcare costs indicated that the cost to avoid one relapse was lower for ozanimod than for the comparator.
Cost Savings
We used an alternative cost-effectiveness approach that focused on cost savings to simultaneously account for relative treatment efficacy and safety, drug costs, and healthcare costs associated with each event of interest. Specifically, a fixed-budget analysis was used to estimate cost savings (or cost offsets) associated with avoiding relapses and AEs for ozanimod vs comparator DMTs. For this analysis, it was assumed that there was an annual fixed budget B (i.e., 1 million US dollars) available for the treatment of patients with RRMS. Assuming also that all patients could be treated with one treatment (either ozanimod or one of the comparators), we calculated the number of patients that could be thus treated for 1 year as the ratio between B and annual drug costs per person. Among treated patients, the number of events (either relapses or AEs) that were prevented was calculated as the total number of treated patients divided by the NNT for avoiding one event with that drug, and this value was used to calculate the difference in relapse events or AEs that were avoided by treating patients with ozanimod vs a comparator. The annual savings from avoiding these events were assumed to be equivalent to healthcare spending associated with their management. Finally, the total savings associated with using ozanimod vs the comparator drug for a budget B were calculated as the sum of savings related to avoiding relapses and savings related to avoiding AEs. Because total savings changed linearly with budget B, the ratio of total savings to B was interpreted as dollars saved per dollar invested, with a larger ratio indicating greater cost-effectiveness of ozanimod vs the comparator. Additional details on these calculations can be found in Online Resource 6 in the electronic supplementary material.
Sensitivity Analysis
Sensitivity analyses were conducted to assess the impact of different values of relapse and AE management costs on (1) total MS-related healthcare costs; (2) incremental MS-related healthcare costs to avoid a relapse; and (3) fixed-cost savings. We considered two scenarios: a high-cost scenario in which the cost of managing a relapse or an SAE was twice the base-case cost and the relative frequency of nonserious AEs vs SAEs was 0.80 (base case = 0.91); and a low-cost scenario in which the cost of managing a relapse or an SAE was half the base-case cost and the relative frequency of nonserious AEs vs SAEs was 0.95. For each treatment, the three outcomes were recalculated on the basis of the costs for each of these two scenarios, and the relative changes compared with the base-case scenario were calculated to quantify the impact of changes in these managing cost estimates.
Results
Incremental Annual Drug Costs Per Relapse Avoided
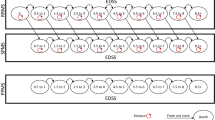
The highest annual drug costs were incurred by patients treated with interferon beta-1a 22 μg and 44 μg ($115,676) and fingolimod ($110,660) (Table 1). The highest drug cost per relapse avoided vs placebo was incurred by patients treated with interferon beta-1a 30 μg ($1,170,022) and teriflunomide 7 mg ($827,458). Compared with other DMTs, ozanimod was associated with lower annual drug costs to avoid a relapse, with incremental cost differences ranging from $823,168 (95% CI − $1,398,077, − $248,259) with interferon beta-1a 30 μg to $72,789 (95% CI − $152,101, $6522) with fingolimod (Fig. 1).
Annual MS-Related Healthcare Costs
Healthcare costs associated with managing a relapse and any AE were $3583 and $1047, respectively. The estimated total annual MS-related healthcare costs per patient ranged from $77,923 for glatiramer acetate 40 mg to $117,570 for interferon beta-1a 44 μg (Fig. 2).
Total MS-Related Healthcare Costs Per Relapse Avoided
Compared with other DMTs, ozanimod was associated with lower total annual healthcare costs to avoid a relapse, with incremental cost differences ranging from $843,684 (95% CI − $1,431,619, − $255,749) with interferon beta-1a 30 μg to $72,847 (95% CI − $153,444, $7750) with fingolimod (Fig. 3 and, in the electronic supplementary material, Online Resource 7).
Cost Savings
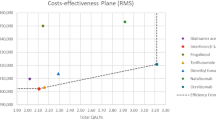
Compared with other DMTs, treatment with ozanimod was associated with annual healthcare cost savings ranging from $2178 (vs fingolimod) to $8257 (vs interferon beta-1a 30 μg) based on a budget of 1 million USD (Fig. 4 and, in the electronic supplementary material, Online Resource 8).
Sensitivity Analyses
The results of the sensitivity analyses showed that the base-case results were robust to relatively large changes in the estimated costs of managing relapses and AEs. Total MS-related healthcare costs were on average 3.8% higher than the base case across all treatments in the high-cost scenario and 1.0% lower in the low-cost scenario. The incremental MS-related healthcare costs per relapse avoided were similar in terms of directionality and statistical significance between the high- and low-cost scenarios compared with the base-case results. The incremental costs were on average 4.0% higher than the base case across all treatments in the high-cost scenario and 1.2% lower in the low-cost scenario. Fixed-cost savings were also qualitatively similar between the high- and low-cost scenarios compared with the base-case results. As expected, the relapse and AE saving components increased in the high-cost scenario and decreased in the low-cost scenario, corresponding to changes in the respective costs of managing each type of event. In terms of total savings (the sum of these two components), the numerical changes were large: total savings were on average 120.4% higher than the base case across all treatments in the high-cost scenario and 51.1% lower in the low-cost scenario (Online Resource 9 in the electronic supplementary material).
Discussion
There are multiple DMTs available for the treatment of RRMS. Although cost alone must never be the sole consideration in therapeutic decision-making, cost-effectiveness analyses that quantify benefits, risks, and costs associated with different treatments are particularly advantageous when the therapeutic armamentarium is wide and clinical guidelines are sparing in their practice recommendations. The current study provides important insights into the clinical and economic impact of oral and injectable DMTs; unlike other cost-effectiveness analyses conducted in North America, this study includes ozanimod, approved in 2020, in the comparison [17,18,19]. First, efficacy and safety data were combined to estimate MS-related healthcare costs. Additionally, a novel cost savings approach was used to compare treatments (i.e., accounting simultaneously for relative treatment efficacy and safety, drug costs, and healthcare costs associated with each outcome of interest). The findings provide healthcare decision makers in the USA with evidence on the cost-effectiveness of DMTs to inform appropriate treatment selection for patients with RRMS.
Our results showed that depending on the DMT, total MS-related healthcare costs per patient per year ranged from approximately $78,000 to $118,000. Compared with glatiramer acetate (20 mg), teriflunomide (7 mg and 14 mg), and interferon beta-1a (30 μg and 44 μg), treatment with ozanimod was associated with significant reductions in annual drug costs and total annual MS-related healthcare costs to avoid relapses. Compared with fingolimod, dimethyl fumarate, and glatiramer acetate (40 mg), ozanimod was associated with numerical reductions in treatment costs and MS-related healthcare costs. In the fixed-budget analysis, ozanimod showed a favorable cost-effectiveness profile compared with other DMTs considering relative treatment efficacy and safety as well as drug and healthcare costs. The fixed-budget analysis highlighted the relative differences between treatment in terms of savings, while the incremental cost per relapse approach highlighted the absolute differences in costs between treatments.
These results were robust to variations in the estimated costs of managing relapses and AEs, particularly for total MS-related healthcare costs, and are in line with a previous analysis demonstrating that ozanimod was associated with a lower cost per relapse avoided compared with dimethyl fumarate, fingolimod, and teriflunomide [28]. In contrast, we used ARR and AE data synthesized via an NMA, which served to adjust for some cross-trial differences by measuring treatment effects relative to a common reference arm. The current study also provided a risk–benefit profile for each treatment by including safety outcomes in addition to ARR and estimated the overall cost savings associated with each treatment by combining incremental drug costs to avoid relapses and AEs along with the associated healthcare costs.
Analyses were conducted to compare incremental drug cost per relapse avoided for ozanimod vs diroximel fumarate (Vumerity), another oral DMT approved by the FDA in 2019 for the treatment of RRMS. The approval of diroximel fumarate was based largely on the safety and efficacy of dimethyl fumarate, given the demonstration of their bioequivalence [29]. Diroximel fumarate was not included in the previous NMA because of a lack of efficacy data. Assuming the same ARR as dimethyl fumarate and an annual drug cost of $88,000, we estimated diroximel fumarate to have an incremental annual drug cost per relapse avoided of − $33,604 (95% CI − $124,285, $57,077) compared with ozanimod, indicating that ozanimod was associated with lower drug cost per relapse avoided compared with diroximel fumarate, although the difference was not statistically significant. However, these results should be interpreted in light of the strong assumptions made regarding the efficacy of diroximel fumarate. Moreover, comparisons were limited to cost per relapse, given the distinct safety profiles of diroximel fumarate and dimethyl fumarate (i.e., AE rates could not be assumed to be the same for the two drugs).
The results of our study should be considered within the context of certain limitations. First, the comparative ARR data were based on indirect evidence synthesized via an NMA because for most DMTs, there were no data from head-to-head comparisons; thus, our analyses may have been biased by unobserved differences between patient populations. Second, the estimation of SAE and AE costs relied on assumptions (i.e., the mix of specific SAEs was assumed to be comparable between different DMTs, and each nonserious AE was assumed to require one outpatient visit and a set of routine laboratory tests) which may not be generalizable to real-world settings. Since the analysis compares the costs associated with treatment over a 1-year period, AEs occurring after treatment discontinuation were not assessed. The approach used to calculate the cost of managing an AE helped to overcome the problem of having a large number of AEs and a lack of outpatient data for specific diagnoses in the Healthcare Cost and Utilization Project data set but was based on the assumption that the relative frequency of SAEs vs nonserious AEs (i.e., the weight w) was similar across DMTs. Moreover, the weight w was calculated by assuming that most patients did not have both serious and nonserious AEs, but an increasing number of patients with both types of AEs would lead to the overestimation of w and consequently of the cost of any AE. It should be noted that the direct and indirect costs of managing relapses may differ between patient populations [30]. To address these limitations, we conducted sensitivity analyses to evaluate the robustness of the main results to variations in the estimated costs of managing AEs. Third, the treatment costs did not include administrative and monitoring costs; however, the latter account for only about 1% of drug costs [28, 31] and are therefore not expected to contribute substantially to differences between treatments. We focused on commonly used first-line therapies for RRMS that are self-administered in order to compare pharmacy costs per relapse avoided. Treatments administered by a healthcare provider (i.e., infusion therapies, which were not considered in this study) may accrue additional costs. Fourth, NMA networks did not include AE data for interferon beta-1a 22 μg and interferon beta-1b 250 μg; therefore, total healthcare cost and fixed-budget analyses that relied on these safety data were not feasible for these treatments. Fifth, our methodology considers treatment effects solely in terms of clinical outcomes reported in clinical trials, rather than benefits measured by patient-reported outcomes or patient preference. Recently, progression independent of relapse activity (PIRA) has been proposed as a mechanism of disability progression in RRMS and even as a uniting feature of MS phenotypes [32,33,34]. However, a universal definition of PIRA has not been validated as an outcome measure or embraced as an endpoint in clinical trials among patients with RRMS, nor was PIRA studied in the clinical trials of the treatments we compared. Finally, it should be noted that the results of this analysis are specific to the USA. However, the methodologic approach, whereby cost-effectiveness was determined using an additional cost per relapse analysis or a fixed-budget analysis could be readily adapted and applied to other healthcare settings with available data.
Conclusion
Compared with all other DMTs evaluated, treatment with ozanimod was associated with reductions in annual drug and total annual MS-related healthcare costs to avoid relapses in patients with RRMS; these reductions were significant versus glatiramer acetate (20 mg), teriflunomide (7 mg and 14 mg), interferon beta-1b (250 μg; annual drug costs only), and interferon beta-1a (30 μg and 44 μg).
References
Stadelmann C. Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr Opin Neurol. 2011;24(3):224–9. https://doi.org/10.1097/WCO.0b013e328346056f.
Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9:S5–48. https://doi.org/10.1016/j.msard.2016.07.003.
Hirst C, Ingram G, Pearson O, et al. Contribution of relapses to disability in multiple sclerosis. J Neurol. 2008;255(2):280–7. https://doi.org/10.1007/s00415-008-0743-8.
Ziemssen T, Derfuss T, de Stefano N, et al. Optimizing treatment success in multiple sclerosis. J Neurol. 2016;263(6):1053–65. https://doi.org/10.1007/s00415-015-7986-y.
Confavreux C, Vukusic S, Moreau T, et al. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430–8. https://doi.org/10.1056/NEJM200011163432001.
Karampampa K, Gustavsson A, Miltenburger C, et al. Treatment experience, burden, and unmet needs (TRIBUNE) in multiple sclerosis study: the costs and utilities of MS patients in Canada. J Popul Ther Clin Pharmacol. 2012;19(1):e11–25.
Nicholas J, Zhou H, Deshpande C. Annual cost burden by level of relapse severity in patients with multiple sclerosis. Adv Ther. 2021;38(1):758–71. https://doi.org/10.1007/s12325-020-01570-0.
Bebo B, Cintina I, LaRocca N, et al. The economic burden of multiple sclerosis in the United States: estimate of direct and indirect costs. Neurology. 2022;98(18):e1810–7. https://doi.org/10.1212/WNL.0000000000200150.
Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med (Lond). 2017;17(6):530. https://doi.org/10.7861/clinmedicine.16-6-s53.
Garg N, Smith TW. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015;5(9):e00362. https://doi.org/10.7861/clinmedicine.16-6-s53.
National Multiple Sclerosis Society. Multiple sclerosis: medications. https://www.nationalmssociety.org/Treating-MS/Medications; Accessed 24 Jun 2022.
Kim Y, Krause TM, Blum P, et al. Disease modifying therapies continue to drive up health care cost among individuals with multiple sclerosis. Mult Scler Relat Disord. 2019;30:69–75. https://doi.org/10.1016/j.msard.2019.02.006.
Tillery EE, Clements JN, Howard Z. What’s new in multiple sclerosis? Ment Health Clin. 2017;7:213–20. https://doi.org/10.9740/mhc.2017.09.213.
Zeposia (ozanimod) capsules, for oral use [prescribing information]. Princeton: Bristol Myers Squibb; revised November, 2022.
Tencer T, Snedecor S, Nicoloso D. Systematic literature review and network meta-analysis of ozanimod compared with other treatments in relapsing-remitting multiple sclerosis. Presented at the 35th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), 11–13 September 2019. Stockholm, Sweden.
Kumar J, Tencer T, Swallow E, et al. Number-needed-to-treat analysis and risk-benefit assessment of ozanimod compared with first-line disease-modifying therapies for relapsing-remitting multiple sclerosis. Presented at the 35th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), 11–13 September 2019. Stockholm, Sweden.
Bozkaya D, Livingston T, Migliaccio-Walle K, et al. The cost-effectiveness of disease-modifying therapies for the treatment of relapsing-remitting multiple sclerosis. J Med Econ. 2017;20(3):297–302. https://doi.org/10.1080/13696998.2016.1258366.
Zimmermann M, Brouwer E, Tice JA, et al. Disease-modifying therapies for relapsing-remitting and primary progressive multiple sclerosis: a cost-utility analysis. CNS Drugs. 2018;32(12):1145–57. https://doi.org/10.1007/s40263-018-0566-9.
Baharnoori M, Bhan V, Clift F, et al. Cost-effectiveness analysis of ofatumumab for the treatment of relapsing-remitting multiple sclerosis in Canada. Pharmacoecon Open. 2022;6(6):859–70. https://doi.org/10.1007/s41669-022-00363-1.
IBM Micromedex®. https://www.micromedexsolutions.com/micromedex2/librarian/ssl/true. Accessed 15 Apr 2019.
Centers for Medicare & Medicaid Services. http://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed 17 Apr 2019.
New Hampshire Employment Security. United States CPI-U. https://www.nhes.nh.gov/elmi/statistics/documents/cpiuscomp.pdf. Accessed 24 Jun 2022.
Nazareth TA, Rava AR, Polyakov JL, et al. Relapse prevalence, symptoms, and health care engagement: patient insights from the Multiple Sclerosis in America 2017 survey. Mult Scler Relat Disord. 2018;26:219–34. https://doi.org/10.1016/j.msard.2018.09.002.
O’Brien JA, Ward AJ, Patrick AR, Caro J. Cost of managing an episode of relapse in multiple sclerosis in the United States. BMC Health Serv Res. 2003;3(1):17. https://doi.org/10.1186/1472-6963-3-17.
O’Connell K, Kelly SB, Fogarty E, et al. Economic costs associated with an MS relapse. Mult Scler Relat Disord. 2014;3(6):678–83. https://doi.org/10.1016/j.msard.2014.09.002.
Oleen-Burkey M, Castelli-Haley J, Lage MJ, Johnson KP. Burden of a multiple sclerosis relapse: the patient’s perspective. Patient. 2012;5(1):57–69. https://doi.org/10.2165/11592160-000000000-00000.
Cohen JA, Comi G, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18(11):1021–33. https://doi.org/10.1016/S1474-4422(19)30238-8.
Nedzesky JHM, Han M, Kamal KM. Cost per relapse avoided of oral therapies in relapsing-remitting multiple sclerosis [abstract G20]. Presented at the AMCP NEXUS 2020 Virtual meeting; October 19, 2020.
National Multiple Sclerosis Society. FDA approves oral Vumerity (diroximel fumarate), similar to Tecfidera, for relapsing MS. 2019. https://www.nationalmssociety.org/About-the-Society/News/FDA-Approves-Oral-Vumerity%E2%84%A2-(Diroximel-Fumarate),#:~:text=Q.,and%20active%20secondary%20progressive%20MS. Accessed 14 Sept 2022.
Ness NH, Schriefer D, Haase R, et al. Real-world evidence on the societal economic relapse costs in patients with multiple sclerosis. Pharmacoeconomics. 2020;38(8):883–92. https://doi.org/10.1007/s40273-020-00917-3.
Mantovani L, Furneri G, Bitonti R, et al. Cost-effectiveness analysis of dimethyl fumarate in the treatment of relapsing remitting multiple sclerosis: an Italian societal perspective. Farmeconomia. 2019;20(1):73–86. https://doi.org/10.7175/fe.v20i1.1437.
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–40. https://doi.org/10.1001/jamaneurol.2020.1568.
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145(9):3147–61. https://doi.org/10.1093/brain/awac016.
Amin M, Hersh CM. Updates and advances in multiple sclerosis neurotherapeutics. Neurodegener Dis Manag. 2023;13(1):47–70. https://doi.org/10.2217/nmt-2021-0058.
Acknowledgements
Funding
This study, its open access publication, and the journal’s Rapid Service Fee were funded by Bristol Myers Squibb.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance were provided by Eleanor Bush, MA, of Peloton Advantage, LLC, an OPEN Health company, and funded by Bristol Myers Squibb.
Author Contributions
Study design: All authors. Formal analysis: Oscar Patterson-Lomba, Elyse Swallow, Akanksha Dua. Data interpretation: All authors. Manuscript writing and editing: All authors.
Prior Presentation
Data were previously presented at the ISPOR Europe 2021 conference, 30 November 2021–3 December 2021 (virtual).
Disclosures
Daniel Kantor has received consulting fees from Biogen, Bristol Myers Squibb, and Janssen. He has received research support from Bristol Myers Squibb. Timothy Pham, now employed at GSK, was an employee of Bristol Myers Squibb when the study was conducted and may be a shareholder in the company. Oscar Patterson-Lomba, Elyse Swallow, and Akanksha Dua are employees of Analysis Group, Inc., a consulting firm that received funding from Bristol Myers Squibb to conduct this research. Komal Gupte-Singh is an employee of Bristol Myers Squibb and may be a shareholder in the company.
Compliance with Ethics Guidelines
Approval from an ethics board was not required to conduct this analysis. The data sets used are publicly available and did not require permission to use. The study did not involve human subjects, and the studies included in the model we report conformed to the Helsinki Declaration of 1964 and its later amendments.
Data Availability
All data generated or analyzed during this study are included and referenced in this article or as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kantor, D., Pham, T., Patterson-Lomba, O. et al. Cost Per Relapse Avoided for Ozanimod Versus Other Selected Disease-Modifying Therapies for Relapsing–Remitting Multiple Sclerosis in the United States. Neurol Ther 12, 849–861 (2023). https://doi.org/10.1007/s40120-023-00463-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00463-y