Abstract
Introduction
Adherence to disease-modifying therapies is key for achieving optimal outcomes in multiple sclerosis (MS). Diroximel fumarate (DRF) is an oral fumarate approved for treatment of relapsing forms of MS. It has the same pharmacologically active metabolite as dimethyl fumarate (DMF) and similar efficacy and safety profiles, but with demonstrated fewer gastrointestinal (GI) related adverse events (AEs). There are limited data characterizing persistence and adherence to DRF in the real world.
Methods
This retrospective analysis of the AcariaHealth Specialty Pharmacy Program included patients with MS initiating DRF from 1 December 2019 to 30 January 2021. This analysis evaluated persistence, measured as proportion of patients remaining on therapy; discontinuation rate due to GI AEs; and adherence measured by proportion of days covered (PDC).
Results
Overall, 1143 patients were included; 433 (37.9%) patients had been treated with prior DMF and switched to DRF. Persistence was high in both groups: the estimated proportion of patients remaining on DRF at 16 months was 82.3% [95% confidence internal (CI) 77.2–86.3%], and 90.1% (95% CI 82.2–94.6%) in the DMF to DRF group. Fifty-two (4.5%) patients overall and 15 (3.5%) in the DMF switch subgroup discontinued DRF due to GI AEs. Mean PDC was 90.8% (95% CI 89.2–92.5%), and 85.4% (95% CI 83.3–87.4%) of patients achieved PDC ≥ 80% in the overall population. In the DMF to DRF group, mean PDC was 90.7% (95% CI 88.0–93.5%), and 84.8% (95% CI 81.4–88.1%) of patients achieved PDC ≥ 80%.
Conclusion
In this analysis of > 1000 patients treated with DRF in real-world clinical practice, overall persistence at 16 months was high, treatment discontinuation due to GI AEs was low, and patients were highly adherent to therapy. Of 433 patients who switched from DMF to DRF, most (> 90%) were able to tolerate and persist on DRF after switching.
Graphical abstract available for this article.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Adherence to disease-modifying therapies is key for achieving optimal outcomes in multiple sclerosis (MS). |
Diroximel fumarate (DRF) is an oral fumarate approved for the treatment of relapsing forms of MS with a low (< 1%) discontinuation rate due to gastrointestinal adverse events (GI AEs) in clinical trials; however, there are limited data characterizing persistence/adherence to DRF in real-world clinical settings. |
This final readout from the retrospective analysis of the AcariaHealth Specialty Pharmacy Program looks at persistence and adherence to DRF in the overall population of > 1000 patients and in a subgroup of patients who have switched from DMF to DRF (n = 433). |
What was learned from the study? |
Overall persistence on DRF at 16 months was high, treatment discontinuation due to GI AEs was low, and patients were highly adherent to therapy. |
Of 433 patients who switched from DMF to DRF, most (> 90%) were able to tolerate and persist on DRF after switching. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.21318204.
Introduction
Treatment with disease-modifying therapies (DMTs) can slow disease progression of multiple sclerosis (MS) by reducing the number and severity of relapses, reducing disease-related disability [1, 2], and slowing or preventing permanent damage to the central nervous system [2, 3]. There are more than 20 DMTs available to treat MS in the USA, including infusions, injectables, and oral DMTs [3]. Adherence to DMT treatment is a key factor for achieving optimal clinical outcomes in MS, owing to the importance of consistent control of disease activity [4, 5] and receiving an effective dose. The most commonly used metric of adherence is proportion of days covered (PDC), and high adherence is defined as a PDC of ≥ 80% in many disease states, including MS [6,7,8,9]. DMT discontinuation is a common challenge, with patients discontinuing treatment for numerous reasons including adverse events (AEs), disease progression, patient perception of drug ineffectiveness, and treatment burden [4, 10, 11]. With an increasingly wide range of treatment options, it is critical to select the optimal therapy for a given patient, considering not only efficacy but also factors that will increase patient adherence and compliance [12, 13].
Diroximel fumarate (DRF) is a next-generation oral fumarate approved in the USA for the treatment of relapsing forms of MS [14] and Europe for the treatment of relapsing–remitting MS [15]. As of 30 June 2022, > 28,000 patients globally have been treated with DRF, representing > 24,000 patient-years of exposure. Of these, 1477 patients (1718 patient-years) were from clinical trials. Oral administration of DRF leads to rapid conversion to monomethyl fumarate (MMF), the same active metabolite as dimethyl fumarate (DMF). At therapeutic doses, DRF and DMF produce bioequivalent systemic exposure of MMF, and therefore are expected to have similar safety and efficacy profiles [11, 16, 17]. MMF is thought to impact MS pathophysiology through antiinflammatory modulation, reducing central nervous system infiltration, and shifting responses from proinflammatory to antiinflammatory [18,19,20]. DMF has demonstrated a favorable benefit–risk profile in both clinical and real-world studies of patients with MS; however, some patients discontinue DMF due to gastrointestinal (GI) AEs, which may develop early in DMF treatment [21,22,23,24,25,26]. DRF has demonstrated reduced incidence and severity of GI AEs as compared with DMF [16, 27, 28], which is hypothesized to be due to its distinct chemical structure; DRF is a larger, more complex molecule and is therefore thought to have less reactivity with off-target proteins. It is also hypothesized to cause less localized irritation in the GI tract, as initial metabolism of DRF causes a significantly lower exposure of methanol compared with DMF [27].
Two phase 3 clinical studies of DRF in patients with MS have demonstrated favorable GI tolerability and low (< 1%) treatment discontinuation due to GI AEs [16, 28]. DRF demonstrated clinically significant improvement in GI tolerability compared with DMF in the 5-week, randomized, head-to-head, phase 3 EVOLVE-MS-2 study, with significantly fewer days of patient-assessed GI symptoms, lower rates of GI AEs, and less treatment discontinuation due to GI AEs (DRF 0.8% versus DMF 4.8%) [16]. In a post hoc analysis of EVOLVE-MS-2, the improved GI profile for DRF compared with DMF was associated with clinically meaningful improvements in quality of life [29]. DRF demonstrated a low rate (0.7%) of discontinuation due to GI AEs in the interim analysis of the 2-year EVOLVE-MS-1 study, which included > 600 patients [28]. A post hoc analysis of EVOLVE-MS-1 showed that patients switching to DRF from DMF or injectable DMTs (glatiramer acetate or interferons) had efficacy and safety data consistent with previous fumarate studies, demonstrating that transition to DRF may be a reasonable treatment strategy for MS patients [11].
Owing to the strict inclusion criteria and structured nature of phase 3 clinical trials, discontinuation rates may not be indicative of rates observed in real-world clinical practice. In previous studies with DMF, the rate of treatment discontinuation due to GI AEs was approximately 4% in randomized, phase 3 clinical trials [17], whereas the rate was higher in real-world studies, varying from 5% to 19% in studies ranging from 3 to 37 months in duration [21, 22, 25, 26, 30,31,32,33,34]. An interim analysis of the AcariaHealth Specialty Pharmacy Program (SPP) including 160 patients with a median (range) DRF treatment duration of 7.6 (0.1–10.4) months showed a discontinuation rate of 3.8% (6/160) due to GI AEs [35]. Although higher than the < 1% discontinuation rate observed in phase 3 DRF clinical trials [16, 28], this was still lower than the observed rate of GI discontinuations in real-world studies with DMF. The interim analysis also demonstrated high adherence to DRF, measured as PDC [35]. However, additional follow-up is needed to evaluate longer-term persistence and adherence to DRF in a larger patient population.
Although GI AEs in patients on DMF most frequently occur in the first 10–12 weeks of treatment, some patients may experience GI AEs that persist longer [31]. These longer-term GI AEs are usually mild-to-moderate in severity and therefore may be less likely to result in patient complaints leading to discontinuation; however, they could impact other treatment outcomes, such as adherence.
Here, we report the final analysis of the retrospective AcariaHealth SPP study, which was designed to evaluate persistence to therapy, discontinuation rates due to GI AEs, and adherence in patients with MS treated with DRF in real-world clinical practice. In addition, this study examined adherence in a subset of patients with GI AEs resulting in discontinuation of DMF ≥ 1 year after initiating DMF.
Methods
Data Source
This is an updated final readout from the retrospective analysis of the AcariaHealth SPP. The study design and study endpoints have been previously described [35]. Permission was obtained from AcariaHealth to access and use the AcariaHealth pharmacy data. This noninterventional study included patients with MS who initiated DRF between 1 December 2019 and 30 January 2021, receiving their prescription from the specialty pharmacy provider AcariaHealth (Troy, MI, USA). Patients were followed until data extraction on 30 June 2021. Patients were excluded if their treatment status could not be determined, such as in the case of patients whose DRF prescription was transferred to a different pharmacy. Information on a patient’s prior DMT use was based on pharmacy records. All patient information was anonymized, and patient confidentiality was maintained through compliance with Health Insurance Portability and Accountability Act regulations. This analysis is based on previously collected data and does not involve any new studies of human subjects performed by any of the authors.
Study Endpoints
Endpoints included persistence, discontinuation rate due to GI AEs, and adherence. Persistence was defined as the overall proportion of patients remaining on therapy. GI AEs included events identified based on pharmacist classification that were directly GI related, in addition to any unknown AE (i.e., an AE lacking details regarding the nature of the event) that occurred within 90 days of initiating DRF therapy. This analytical approach was used to avoid underestimation of the GI AE discontinuation rate. If patients were classified as being discontinued due to an AE, they were stratified as either GI AE or “other AE.” Information on AEs and reasons for treatment discontinuation were collected prior to each prescription refill and recorded in the pharmacy database by AcariaHealth pharmacy staff. Adherence was measured by the PDC; this was calculated by dividing the number of days in the treatment period that a patient is ‘‘covered’’ by having medication on hand by the total number of days in the treatment period, and then multiplying by 100. The number of days a patient is covered by having medication on hand is based on pharmacy records of each time a patient requested a refill of their prescription.
Statistical Analysis
Outcomes were evaluated in the overall population and in a subgroup of patients who received DMF as the most recent DMT before switching (DMF to DRF switch). Descriptive statistics were used to summarize demographic and disease characteristics of the study population. Continuous variables were summarized using the mean [standard deviation (SD)] or median (range) as appropriate, and categorical variables were summarized using frequency (percentage). Persistence was characterized using the Kaplan–Meier method with 95% confidence intervals (CIs). Although some patients were treated for up to 20 months, the Kaplan–Meier estimate for DRF persistence was reported for up to 16 months to ensure a meaningful sample size. Discontinuation rate and PDC were also characterized with 95% CIs. As it was possible that healthcare providers (HCPs) may prescribe an extended titration period (beyond the USA prescribing information defined 1-week titration for DRF), a PDC sensitivity analysis was conducted that excluded the first month (the first DRF shipment) from the PDC calculation (sensitivity analysis 1). To determine whether the PDC could be impacted by patients who have been on treatment for < 6 months, we conducted a second sensitivity analysis evaluating PDC in a subgroup of patients who were treated with DRF for ≥ 6 months (sensitivity analysis 2).
Finally, adherence before and after switching to DRF was evaluated in a subgroup of patients who had lingering GI AEs; “lingering GI AEs” were defined as GI AEs resulting in discontinuation of DMF ≥ 1 year after initiating DMF.
The raw data set was prepared using SQL Server Management Studio. A comprehensive SQL script was created to supply all of the identified demographic values for the study, along with the measures necessary to calculate the study endpoints. Using the SQL Script output, data were loaded into Microsoft Excel for analysis.
Results
Study Population
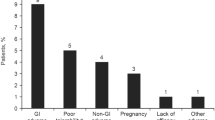
Overall, 1143 patients with MS were included in the analysis. The median (range) age at enrollment was 51 (19–83) years, and 75.2% (860/1143) were women (Table 1). The median (range) DRF treatment duration was 7.1 (0.1–20.0) months (Table 2). Of the overall study population, 60.3% (689/1143) had no prior DMT based on the pharmacy records, while 37.9% (433/1143) had received prior DMF treatment and were included in the DMF to DRF subgroup. The mean (range) age at enrollment in this subgroup was 55 (22–83) years, and 75.5% (327/433) were women. Patients had been treated with prior DMF for a median (range) of 13.7 (0.2–91.6) months before they switched to DRF. After switching to DRF, the median (range) DRF treatment duration was 6.9 (0.6–18.6) months. Of those with a known reason for discontinuing DMF (89/433; 20.6%), 37 (41.6%) discontinued due to GI AEs.
Persistence and Adherence in Overall Population and DMF to DRF Subgroup
The estimated proportion of patients remaining persistent on DRF treatment at 16 months was 82.3% (95% CI 77.2–86.3%) in the overall population and 90.1% (95% CI 82.2–94.6%; Fig. 1) in the DMF to DRF subgroup. The rate of DRF discontinuation due to GI AEs was low in both groups: 4.5% (52/1143) in the overall population and 3.5% (15/433) in the DMF to DRF subgroup (Table 3). In the overall population, mean PDC was 90.8% (95% CI 89.2–92.5%; Fig. 2a), and the proportion of patients with PDC ≥ 80% was 85.4% (95% CI 83.3–87.4%; Fig. 2b). Adherence was consistently > 90% regardless of which region of the USA the patients resided: 90.2% PDC for Northeast; 91.5% PDC for Midwest; 90.1% PDC for South; 91.7% PDC for West. Mean PDC was 90.7% (95% CI 88.0–93.5%; Fig. 3a) in the DMF to DRF subgroup, and the proportion of patients with PDC ≥ 80% was 84.8% (95% CI 81.4–88.1%; Fig. 3b). In both populations, PDC remained high when excluding the first DRF refill to account for extended titration (sensitivity analysis 1), with mean PDC 94.9% (95% CI 93.7–96.2%) in the overall population and 95.7% (95% CI 93.7–97.6%) in the DMF to DRF subgroup. PDC also remained high in the subgroup of patients treated for ≥ 6 months (sensitivity analysis 2) in both the overall and DMF to DRF populations, with mean PDC 90.2% (95% CI 88.2–92.3%) and 90.2% (95% CI 87.0–93.4%), respectively.
Persistence to DRFa in the overall study population and DMF to DRF subgroup. aPersistence was characterized using the Kaplan–Meier method with 95% CIs (95% CI indicated by shaded area). Although some patients were treated for up to 20 months, the Kaplan–Meier estimate for DRF persistence was reported to 16 months to ensure a meaningful sample size. Patient numbers beyond 16 months are too small to yield a reliable estimate. CI confidence interval, DMF dimethyl fumarate, DRF diroximel fumarate
Adherence to DRF in the overall study population. aSensitivity analysis 1: excluding first DRF fill to account for healthcare provider–prescribed extended titration regimens. bSensitivity analysis 2: subgroup of patients with ≥ 6 months of treatment duration. CI confidence interval, DMF dimethyl fumarate, DRF diroximel fumarate, PDC proportion of days covered
Adherence to DRF in the DMF to DRF subgroup. aSensitivity analysis 1: excluding first DRF fill to account for healthcare provider–prescribed extended titration regimens. bSensitivity analysis 2: subgroup of patients with ≥ 6 months of treatment duration. CI confidence interval, DMF dimethyl fumarate, DRF diroximel fumarate, PDC proportion of days covered
Persistence in Patients with Lingering GI AEs on DMF
Lingering GI AEs were defined as GI AEs that resulted in discontinuation of DMF > 1 year after DMF initiation. In the DMF to DRF subgroup, a total of 18 patients met the criteria for having lingering GI AEs leading to discontinuation of DMF. Among these 18 patients, the median duration of prior DMF was 28 months. After switching to DRF, most of these patients remained persistent on DRF [16/18 (89%)]; two of these patients discontinued treatment 18 days after DRF initiation due to GI AEs on DRF. Mean PDC increased following the switch from DMF to DRF, from 71% (95% CI 59.1–83.0%) while on DMF to 92.9% (95% CI 88.6–97.2%; p = 0.002, n = 18) on DRF (Fig. 4). Proportion of patients with PDC ≥ 80% also increased following the switch from DMF to DRF, from 44.4% (95% CI 21.5–67.4%) to 94.4% (95% CI 83.9–100.0%; p = 0.001, n = 18) on DRF.
Adherence before and after switching to DRF in patients with lingering GI AEs on DMF (n = 18)a. aLingering GI AEs were defined as those GI AEs resulting in discontinuation of DMF ≥ 1 year after initiating DMF. AE adverse event, CI confidence interval, DMF dimethyl fumarate, DRF diroximel fumarate, GI gastrointestinal, PDC proportion of days covered
Discussion
Persistence and adherence to DRF was high in both the overall population and the DMF to DRF switch subgroup
In this updated real-world analysis, > 1000 patients with MS were treated with DRF. Overall, persistence was high, discontinuation rate due to GI AEs was low, and patients were highly adherent to therapy. This is consistent with the interim AcariaHealth SPP analysis [35] and with the GI tolerability profile shown in clinical trials [11, 16, 28]. Discontinuation of DRF due to GI AEs in this study was 4.5% in the overall population and 3.5% in the DMF to DRF switch subgroup. Although the phase 3 EVOLVE-MS-1 study reported a lower discontinuation rate due to GI AEs in patients treated with DRF (< 1%) [11, 16, 28], a similar trend was observed with DMF, where discontinuation rates increased three- to fourfold in real-world studies compared with clinical trials. In DMF studies, the GI-related discontinuation rate was 5–19% in real-world studies compared with approximately 4% in clinical trials [21, 22, 25, 30,31,32].
A previous retrospective study using US claims databases, reported PDCs of 68.2% for teriflunomide, 71.0% for DMF, and 81.4% for fingolimod at 1 year of treatment [36]. The high rate of adherence with DRF (mean PDC 90.8%) in our study demonstrated that patients had good therapeutic coverage with infrequent gaps in therapy fulfillment. PDC was also high in the DMF to DRF switch subgroup (90.7%), suggesting that switching to DRF is a viable treatment strategy for patients on DMF.
Some HCPs may prescribe an extended titration period (beyond the US prescribing information 1-week titration for DRF) when initiating patients on DMF/DRF [37]. This was accounted for in sensitivity analysis 1 by excluding the first prescription from the PDC calculation, and there was a slight increase in mean PDC to 94.9% in the overall population and 95.7% in the DMF to DRF switch population. PDC was also high in patients with a DRF treatment of ≥ 6 months, suggesting that patients can maintain the two-capsule, twice-daily dosing regimen, and that this pill burden does not negatively affect adherence and persistence.
High adherence to DMTs is an important factor in achieving optimal outcomes in MS treatment, and previous studies have demonstrated that patients with MS who were more adherent to DMTs were at a lower risk of relapse and MS-related inpatient hospitalizations, had fewer care or physician visits, and had lower MS-related costs [38,39,40]. Although consensus on what is deemed an acceptable level of adherence has not been reached, a PDC ≥ 80% is generally considered to be adherent [6, 8, 41]. Using PDC ≥ 80% as the standard, adherence to DRF in this study remained high: 85.4% of patients in the overall population, 94.3% of patients in the analysis excluding the first prescription, and 84.6% of patients who had remained on DRF ≥ 6 months had a PDC ≥ 80%. Similar values for percentage of patients with PDC ≥ 80% were recorded in the subgroup of patients switching from DMF to DRF.
After switching to DRF, adherence improved in DMF treated patients who had lingering GI AEs
Adherence in a subset of patients (n = 18) with lingering GI AEs on DMF increased significantly when they switched to DRF. Mean PDC in this group increased from 71.1% on DMF to 92.9% on DRF, while the percentage of patients with PDC ≥ 80% increased from 44.4% on DMF to 94.4% on DRF. Thirty-seven (41.6%) of the 89 patients with a known reason for discontinuing DMF discontinued due to GI AEs; however, this may be underrepresented as 344 (79.4%) patients had an unknown reason for discontinuing treatment with DMF. These data suggest that switching patients to DRF may be a viable strategy for improving treatment adherence in patients with lingering GI AEs on DMF; however, interpretation of this subset analysis is limited due to the small sample size of patients with lingering GI AEs on DMF. Additional follow-up is warranted to further characterize this specific subgroup.
Limitations
While furthering this research by including comparative analyses with other DMTs would be worthwhile, the AcariaHealth SPP lacks detailed baseline characteristics available through other types of data sources, such as a retrospective chart review, limiting its use for comparative studies that require baseline data to adjust for differences between comparator groups.
The scope of the study included systemic evaluation of GI-related AEs, as those were AEs of interest based on the clinical development of DRF and the previous phase 3 study demonstrating differentiated GI profile for DRF [16]. Therefore, this study was not designed to systematically evaluate other AEs, though this could be done in future analyses of the AcariaHealth Specialty Pharmacy. The study was also limited to information captured by the pharmacy database; for example, it is likely the number of patients DMT-naïve is lower than the reported 60.3%, as some patients may have been treated with a prior DMT that was not captured in the pharmacy database. Nevertheless, this study provides valuable information on DRF, as there is limited real-world data presently available for DRF. Furthermore, although these data lack some of the granular information that could be captured in a medical chart review study, medical chart reviews would likely have smaller patient numbers than those included in this analysis.
It is possible that the discontinuations due to GI AEs may have been overreported in this study, as GI AEs included any unknown AE (i.e., an AE lacking details regarding the nature of the event) that occurred within 90 days of initiating DRF therapy. However, this approach was used to avoid underestimation of the GI AE discontinuation rate, and because GI AEs that occurred in patients taking DMF typically occurred in the first 10–12 weeks of treatment [31].
In addition, PDC as a measure of adherence has limitations, as it measures timely refilling and a patient’s access to a drug, but it cannot definitively determine if a patient is taking each dose of medication as directed; this limitation is not unique to PDC, as it applies to most measures of adherence, including pill counting. PDC was used to measure adherence in this study rather than medication possession ratio (MPR), as MPR represents the sum of days’ supply for all prescription fills relative to the number of days in the treatment period. This means that if the patient obtains medication earlier than required, the MPR could be > 100%, providing a measurement that overestimates adherence. Using PDC eliminates this possibility. Furthermore, the AcariaHealth SPP does not automatically ship DRF refills to patients; instead, they require that patients indicate when the next refill is needed, making PDC a reasonable estimate of adherence for this study. Despite its limitations, PDC is widely accepted as a valid measure of patient adherence and is the preferred method for assessing adherence by the Pharmacy Quality Alliance for use in the Medicare plan Star Ratings [42].
Finally, it is important to note that the median age of patients in the overall population of this study was 51 years old, and patients ranged from 19 to 83 years of age. This is an older population than typically seen in clinical trials, with previous DRF trials having a median age of approximately 40 years old [11, 16, 28]. The difference in age is likely due to this being a real-world study, whereas clinical trials typically set an upper age limit. Although the effect of age on adherence to treatment is not known, these data reflect the use of DRF in real-world clinical practice.
Conclusions
In this updated analysis of more than 1000 patients treated with DRF in real-world clinical practice, overall persistence was high, treatment discontinuation due to GI AEs was low, and patients were highly adherent to therapy. In a subgroup of patients who switched from DMF to DRF, most patients (> 90%) were able to tolerate DRF after switching, and these patients had a high rate of adherence consistent with the overall population. In patients who experienced lingering GI AEs on DMF and subsequently switched to DRF, most (89%) remained persistent to DRF after switching, and medication adherence significantly increased after switching from DMF to DRF.
References
Boster A, Nicholas J, Wu N, et al. Comparative effectiveness research of disease-modifying therapies for the management of multiple sclerosis: analysis of a large health insurance claims database. Neurol Ther. 2017;6(1):91–102. https://doi.org/10.1007/s40120-017-0064-x.
Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med (Lond). 2016;16(Suppl 6):s53–9. https://doi.org/10.7861/clinmedicine.16-6-s53.
National MS Society: Disease-modifying therapies for MS. https://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Brochure-The-MS-Disease-Modifying-Medications.pdf (2021). Accessed 16 May 2022.
Hillert J, Magyari M, Soelberg Sorensen P, et al. Treatment switching and discontinuation over 20 years in the Big Multiple Sclerosis Data Network. Front Neurol. 2021;12: 647811. https://doi.org/10.3389/fneur.2021.647811.
Lizan L, Comellas M, Paz S, Poveda JL, Meletiche DM, Polanco C. Treatment adherence and other patient-reported outcomes as cost determinants in multiple sclerosis: a review of the literature. Patient Prefer Adherence. 2014;8:1653–64. https://doi.org/10.2147/PPA.S67253.
Boland MR, van Boven JF, Kruis AL, et al. Investigating the association between medication adherence and health-related quality of life in COPD: methodological challenges when using a proxy measure of adherence. Respir Med. 2016;110:34–45. https://doi.org/10.1016/j.rmed.2015.11.008.
Garcia-Sempere A, Hurtado I, Sanfelix-Genoves J, Rodriguez-Bernal C, Peiro S, Sanfelix-Gimeno G. Improving the accuracy of medication adherence measures using linked prescription and dispensation data: findings from the ESOSVAL cohort of patients treated with osteoporosis drugs. Curr Med Res Opin. 2019;35(9):1535–44. https://doi.org/10.1080/03007995.2019.1601944.
Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303–10. https://doi.org/10.1185/03007990903126833.
Nicholas JA, Edwards NC, Edwards RA, Dellarole A, Grosso M, Phillips AL. Real-world adherence to, and persistence with, once- and twice-daily oral disease-modifying drugs in patients with multiple sclerosis: a systematic review and meta-analysis. BMC Neurol. 2020;20(1):281. https://doi.org/10.1186/s12883-020-01830-0.
Daugherty KK, Butler JS, Mattingly M, Ryan M. Factors leading patients to discontinue multiple sclerosis therapies. J Am Pharm Assoc (2003). 2005;45(3):371–5. https://doi.org/10.1331/1544345054003804.
Wray S, Then Bergh F, Wundes A, et al. Efficacy and safety outcomes with diroximel fumarate after switching from prior therapies or continuing on DRF: results from the phase 3 EVOLVE-MS-1 study. Adv Ther. 2022;39(4):1810–31. https://doi.org/10.1007/s12325-022-02068-7.
Cree BAC, Mares J, Hartung HP. Current therapeutic landscape in multiple sclerosis: an evolving treatment paradigm. Curr Opin Neurol. 2019;32(3):365–77. https://doi.org/10.1097/WCO.0000000000000700.
Lee Mortensen G, Rasmussen PV. The impact of quality of life on treatment preferences in multiple sclerosis patients. Patient Prefer Adherence. 2017;11:1789–96. https://doi.org/10.2147/PPA.S142373.
Biogen: Vumerity Prescribing Information. https://www.vumerityhcp.com/content/dam/commercial/vumerity/hcp/en_us/pdf/vumerity-prescribing-information.pdf (2020). Accessed 9 Aug 2022.
B.V. BN: VUMERITY® Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/vumerity-epar-product-information_en.pdf (2022). Accessed July 22 2022.
Naismith RT, Wundes A, Ziemssen T, et al. Diroximel fumarate demonstrates an improved gastrointestinal tolerability profile compared with dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: results from the randomized, double-blind, phase III EVOLVE-MS-2 study. CNS Drugs. 2020;34(2):185–96. https://doi.org/10.1007/s40263-020-00700-0.
Biogen: Tecfidera Prescribing Information. https://www.tecfidera.com/content/dam/commercial/multiple-sclerosis/tecfidera/pat/en_us/pdf/full-prescribing-info.pdf (2019). Accessed 9 Apr 2019.
Chen H, Assmann JC, Krenz A, et al. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest. 2014;124(5):2188–92. https://doi.org/10.1172/JCI72151.
Parodi B, Rossi S, Morando S, et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 2015;130(2):279–95. https://doi.org/10.1007/s00401-015-1422-3.
Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, et al. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci USA. 2016;113(17):4777–82. https://doi.org/10.1073/pnas.1603907113.
Chinea A, Amezcua L, Vargas W, et al. Real-world safety and effectiveness of dimethyl fumarate in Hispanic or Latino patients with multiple sclerosis: 3-year results from ESTEEM. Neurol Ther. 2020;9(2):495–504. https://doi.org/10.1007/s40120-020-00192-6.
Fox RJ, Miller DH, Phillips JT, et al.; CONFIRM Study Investigators. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–97. https://doi.org/10.1056/NEJMoa1206328.
Gold R, Arnold DL, Bar-Or A, et al. Safety and efficacy of delayed-release dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: 9 years’ follow-up of DEFINE, CONFIRM, and ENDORSE. Ther Adv Neurol Disord. 2020;13:1756286420915005–05. https://doi.org/10.1177/1756286420915005.
Gold R, Arnold DL, Bar-Or A, et al. Long-term safety and efficacy of dimethyl fumarate for up to 13 years in patients with relapsing-remitting multiple sclerosis: final ENDORSE study results. Mult Scler. 2022;28(5):801–16. https://doi.org/10.1177/13524585211037909.
Williams MJ, Amezcua L, Okai A, et al. Real-world safety and effectiveness of dimethyl fumarate in Black or African American patients with multiple sclerosis: 3-year results from ESTEEM. Neurol Ther. 2020;9(2):483–93. https://doi.org/10.1007/s40120-020-00193-5.
Mallucci G, Annovazzi P, Miante S, et al. Two-year real-life efficacy, tolerability and safety of dimethyl fumarate in an Italian multicentre study. J Neurol. 2018;265(8):1850–9. https://doi.org/10.1007/s00415-018-8916-6.
Palte MJ, Wehr A, Tawa M, et al. Improving the gastrointestinal tolerability of fumaric acid esters: early findings on gastrointestinal events with diroximel fumarate in patients with relapsing-remitting multiple sclerosis from the phase 3, open-label EVOLVE-MS-1 study. Adv Ther. 2019;36(11):3154–65. https://doi.org/10.1007/s12325-019-01085-3.
Naismith RT, Wolinsky JS, Wundes A, et al. Diroximel fumarate (DRF) in patients with relapsing-remitting multiple sclerosis: Interim safety and efficacy results from the phase 3 EVOLVE-MS-1 study. Mult Scler. 2020;26(13):1729–39. https://doi.org/10.1177/1352458519881761.
Wundes A, Wray S, Gold R, et al. Improved gastrointestinal profile with diroximel fumarate is associated with a positive impact on quality of life compared with dimethyl fumarate: results from the randomized, double-blind, phase III EVOLVE-MS-2 study. Ther Adv Neurol Disord. 2021;14:1756286421993999. https://doi.org/10.1177/1756286421993999.
Kresa-Reahl K, Repovic P, Robertson D, Okwuokenye M, Meltzer L, Mendoza JP. Effectiveness of delayed-release dimethyl fumarate on clinical and patient-reported outcomes in patients with relapsing multiple sclerosis switching from glatiramer acetate: RESPOND, a prospective observational study. Clin Ther. 2018;40(12):2077–87. https://doi.org/10.1016/j.clinthera.2018.10.011.
Min J, Cohan S, Alvarez E, et al. Real-world characterization of dimethyl fumarate-related gastrointestinal events in multiple sclerosis: management strategies to improve persistence on treatment and patient outcomes. Neurol Ther. 2019;8(1):109–19. https://doi.org/10.1007/s40120-019-0127-2.
Vollmer B, Nair KV, Sillau SH, Corboy J, Vollmer T, Alvarez E. Comparison of fingolimod and dimethyl fumarate in the treatment of multiple sclerosis: two-year experience. Mult Scler J Exp Transl Clin. 2017;3(3):2055217317725102. https://doi.org/10.1177/2055217317725102.
Mirabella M, Prosperini L, Lucchini M, et al. Safety and efficacy of dimethyl fumarate in multiple sclerosis: an Italian, multicenter real-world study. CNS Drugs. 2018;32(10):963–70. https://doi.org/10.1007/s40263-018-0543-3.
Pilo de la Fuente B, Sabin J, Galan V, et al. Three-year effectiveness of dimethyl fumarate in multiple sclerosis: a prospective multicenter real-world study. CNS Drugs. 2020;34(12):1275–86. https://doi.org/10.1007/s40263-020-00775-9.
Liseno J, Lager B, Miller C, Shankar SL, Mendoza JP, Lewin JB. Multiple sclerosis patients treated with diroximel fumarate in the real-world setting have high rates of persistence and adherence. Neurol Ther. 2021. https://doi.org/10.1007/s40120-021-00242-7.
Johnson KM, Zhou H, Lin F, Ko JJ, Herrera V. Real-world adherence and persistence to oral disease-modifying therapies in multiple sclerosis patients over 1 year. J Manag Care Spec Pharm. 2017;23(8):844–52. https://doi.org/10.18553/jmcp.2017.23.8.844.
Phillips JT, Selmaj K, Gold R, et al. Clinical significance of gastrointestinal and flushing events in patients with multiple sclerosis treated with delayed-release dimethyl fumarate. Int J MS Care. 2015;17(5):236–43. https://doi.org/10.7224/1537-2073.2014-069.
Gerber B, Cowling T, Chen G, Yeung M, Duquette P, Haddad P. The impact of treatment adherence on clinical and economic outcomes in multiple sclerosis: real world evidence from Alberta. Canada Mult Scler Relat Disord. 2017;18:218–24. https://doi.org/10.1016/j.msard.2017.10.001.
Menzin J, Caon C, Nichols C, White LA, Friedman M, Pill MW. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm. 2013;19(1 Suppl A):S24–40. https://doi.org/10.18553/jmcp.2013.19.s1.S24.
Tan H, Cai Q, Agarwal S, Stephenson JJ, Kamat S. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther. 2011;28(1):51–61. https://doi.org/10.1007/s12325-010-0093-7.
Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251–60. https://doi.org/10.2147/CEOR.S130334.
Health Mart Systems ISRR: Star ratings: what pharmacists need to know. https://join.healthmart.com/clinical-performance/star-ratings-what-pharmacists-need-to-know/ (2017). Accessed 28 Oct 2020.
Acknowledgements
Funding
This study was sponsored by Biogen (Cambridge, MA, USA). Biogen also provided funding for the journal’s Rapid Service Fee.
Medical Writing, Editorial, and Other Assistance
Biogen provided funding for medical writing and editorial support in the development of this manuscript; Annabel Campbell, PhD (Excel Medical Affairs), wrote the first draft of the manuscript based on input from authors and Cara Farrell (Excel Medical Affairs) copyedited and styled the manuscript per journal requirements.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All named authors contributed to the design of the study. Data collection was performed by Jacob Liseno and Brittney Lager. Data analysis and interpretation was performed by all authors. Jacob Liseno performed the statistical analyses. All authors participated in drafting/critically revising the manuscript.
Disclosures
Brittney Lager and Jacob Liseno are employees of AcariaHealth. Ivan Božin, Sarah M. England, Sai L. Shankar, Jason P. Mendoza, and James B. Lewin are employees of and hold stock/stock options in Biogen.
Compliance with Ethics Guidelines
Permission was obtained from AcariaHealth to access and use the AcariaHealth pharmacy data. All patient information was anonymized, and patient confidentiality was maintained through compliance with Health Insurance Portability and Accountability Act regulations. This analysis is based on previously collected data and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lager, B., Liseno, J., Božin, I. et al. Real-World Analysis Affirms the High Persistence and Adherence Observed with Diroximel Fumarate in Patients with Multiple Sclerosis. Neurol Ther 12, 145–159 (2023). https://doi.org/10.1007/s40120-022-00413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00413-0