Abstract
Introduction
This randomized, double-blind, placebo-controlled study in healthy volunteers assessed the safety, tolerability, and pharmacokinetics of single ascending doses of intravenously administered NX210—a linear peptide derived from subcommissural organ-spondin—and explored the effects on blood/urine biomarkers and cerebral activity.
Methods
Participants in five cohorts (n = 8 each) were randomized to receive a single intravenous dose of NX210 (n = 6 each) (0.4, 1.25, 2.5, 5, and 10 mg/kg) or placebo (n = 2 each); in total, 10 and 29 participants received placebo and NX210, respectively. Blood samples were collected for pharmacokinetics within 180 min post dosing. Plasma and urine were collected from participants (cohorts: 2.5, 5, and 10 mg/kg) for biomarker analysis and electroencephalography (EEG) recordings within 48 h post dosing. Safety/tolerability and pharmacokinetic data were assessed before ascending to the next dose.
Results
The study included 39 participants. All dosages were safe and well tolerated. All treatment-emergent adverse events (n = 17) were of mild severity and resolved spontaneously (except one with unknown outcome). Twelve treatment-emergent adverse events (70.6%) were deemed drug related; seven of those (58.3%) concerned nervous system disorders (dizziness, headache, and somnolence). The pharmacokinetic analysis indicated a short half-life in plasma (6–20 min), high apparent volume of distribution (1870–4120 L), and rapid clearance (7440–16,400 L/h). In plasma, tryptophan and homocysteine showed dose-related increase and decrease, respectively. No drug dose effect was found for the glutamate or glutamine plasma biomarkers. Nevertheless, decreased blood glutamate and increased glutamine were observed in participants treated with NX210 versus placebo. EEG showed a statistically significant decrease in beta and gamma bands and a dose-dependent increasing trend in alpha bands. Pharmacodynamics effects were sustained for several hours (plasma) or 48 h (urine and EEG).
Conclusion
NX210 is safe and well tolerated and may exert beneficial effects on the central nervous system, particularly in terms of cognitive processing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Progression of dementia negatively affects cognitive processing; it is expected that the number of patients with dementia worldwide will reach 152 million by the middle of the century, partly owing to the extended life span of individuals. |
Available drugs targeting cognitive processing only temporarily delay the occurrence of symptoms. There is an urgent need to accelerate the development of new therapeutics against these neurological diseases by simultaneously targeting several underlying pathophysiological mechanisms. |
This is a first-in-human exploratory study assessing the safety, tolerability, and pharmacokinetics of a new compound (subcommissural organ-spondin-derived peptide NX210) that targets central nervous system diseases/disorders, in particular those associated with cognitive dysfunction. |
What was learned from the study? |
In this single ascending dose study, intravenous administration of NX210 in adult healthy volunteers showed a good safety and tolerability profile, regardless of the dose. |
NX210 metabolite (NX210c) was rapidly eliminated in the systemic circulation (half-life 6–20 min), and the pharmacodynamic variations were sustained (several hours in plasma, up to 48 h in urine and electroencephalography). |
NX210c may decrease the levels of homocysteine in the plasma, which is a known independent risk factor for neurovascular diseases. Its impact on electroencephalography bands is compatible with that of cognitive enhancers. |
The present analysis provides preliminary evidence concerning the effect of NX210/NX210c, a new peptide with multiple properties, on the central nervous system and on cognitive processing in particular. |
Introduction
The prevalence of neurocognitive disorders, including neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PaD), is increasing, partly as a result of the extended life span of individuals. It is expected that the number of patients with dementia worldwide will reach 152 million by the middle of the century, particularly in low- and middle-income countries [1]. In addition to these neurodegenerative diseases, the ongoing coronavirus disease-2019 (COVID-19) pandemic is expected to trigger cognitive decline and dementia [2], thereby markedly increasing the number of patients with dementia in the long term [3]. Cognitive disorders refer to a category of mental health conditions that primarily affect the learning, memory, perception, and problem-solving abilities of patients. AD is the most common cause of dementia, accounting for up to 80% of all dementia diagnoses. The annual direct/indirect healthcare costs related to AD are estimated at nearly US $500 billion [4]. Vascular dementia is the second most common form of dementia after AD. Vascular cognitive disorders are a heterogeneous group of neurocognitive disorders characterized by cognitive decline, which is primarily attributed to cerebrovascular diseases [5, 6]. PaD is another prevalent neurodegenerative disease and the second most ubiquitous age-related disorder, affecting more than 1% of the population aged 65 years and older. Cognitive impairment is up to six times more common in patients with PaD versus healthy individuals and is a very important non-motor manifestation of PaD [7].
Available drugs targeting cognitive impairment in neurocognitive diseases only temporarily delay the occurrence of symptoms and ultimately fail to alter the disease course. Considerable efforts have been made for the discovery of new treatments, and several new compounds have been developed over the past decades. However, most candidate compounds failed to show efficacy in clinical studies. Aducanumab—a new amyloid beta-directed antibody—received conditional accelerated approval by the US Food and Drug Administration (FDA) in June 2021. However, it was not approved by the European Medicines Agency in December 2021. This discrepancy in the approval process emphasizes the risks associated with AD and the urgent need for novel treatment(s) involving new mechanisms of action. Hence, a new therapeutic approach and current utmost challenge is to identify drugs that can exert their effects against these neurological diseases by targeting several underlying pathophysiological mechanisms. The goal of this approach would be to treat symptoms, as well as modify the disease course.
NX210 is a chemically synthesized dodecapeptide derived from the subcommissural organ (SCO)-spondin—a large multi-domain glycoprotein composed of 4500 amino acids—specific to the central nervous system (CNS) extracellular matrix. NX210 is secreted by specialized ependymocytes located in the SCO, a highly conserved structure located in the roof of the third brain ventricle of all vertebrates [8] that plays a role in neurogenesis and axonal guidance during embryogenesis. Owing to oxidation and the consequent formation of an intrachain disulfide bridge between the two cysteines, the peptide can take a cyclic conformation and is termed cyclic NX210 (NX210c). Preclinical in vitro and in vivo data have shown that NX210 exhibits important properties, which may be suitable for the treatment of neurological disorders in humans (i.e., neuroprotection, neuroregeneration, induction of remodeling and cellular plasticity mechanisms, and anti-neuroinflammatory action) [8, 9]. This includes data showing that NX210/NX210c prevents glutamate-related cell death (excitotoxicity) in various primary neuronal cell types of human or rodent origin [10]. Preclinically, NX210/NX210c reduces common hallmarks of AD pathology and restores learning and memory at both early and late stages of the disease. As recently reported, in a mouse model of AD induced by the intracerebroventricular injection of Aβ25–35 oligomers, NX210/NX210c fully restored spatial working and contextual long-term memories. This therapeutic effect was sustained for up to 4 months [9]. Owing to its pleiotropic properties, NX210 targets cognitive disorders (e.g., neurodegenerative diseases) [9] as well as CNS injuries (e.g., traumatic brain or spinal cord injuries) [8]. In addition, a CNS biodistribution study following a single intravenous administration of [3H]NX210c to male non-pigmented rats suggested that NX210c passes through the blood–cerebrospinal fluid (CSF) barrier. This observation was based on the presence of radioactivity in the CSF, cisterna magna, and the choroid plexus at 15 and 30 min after dosing (unpublished data).
Collectively, these results highlight the potential effectiveness of NX210/NX210c in CNS diseases and emphasizes the need for clinical investigations on its use as a therapeutic agent against neurodegenerative diseases, including cognitive disorders. Although the precise mechanism underlying the action of NX210 remains to be determined, the aforementioned preclinical results supported the investigation of this new compound in humans.
The objective of this first-in-human study was to investigate parameters concerning the safety/tolerability (e.g., adverse events [AE), laboratory findings, 12-lead electrocardiography), pharmacokinetics (PK) (e.g., half-life, apparent total clearance, volume of distribution), and pharmacodynamics (PD) (biomarkers in blood and urine and changes in electroencephalography [EEG]) profiles induced by NX210.
Methods
Study Design
This was a single-center, randomized, placebo-controlled, double-blind, single ascending dose study performed from 10 June 2020 to 1 November 2020. Before the study was initiated, the clinical trial protocol, participant information, and informed consent forms were approved by the Independent Ethics Committee of the trial center (Medisch Ethische Toetsingscommissie Assen) on 12 May 2020 (CCMO code NL73571.056.20; Study code 190166-CS0332). The principles of informed consent were implemented according to the latest version of the Declaration of Helsinki, the International Conference on Harmonization Guideline for Good Clinical Practice, and regulatory requirements (Wet medisch-wetenschappelijk onderzoek met mensen, Nederland). The EudraCT registration identifier for the trial was 2020-000859-12.
The study was monitored by a safety review committee (SRC). The intent of the SRC was to ensure that treatment did not pose undue risk to participants. Safety, tolerability, and PK data from at least six evaluable participants per cohort were assessed by the SRC within each cohort before ascending to the next dose level. SRC meetings occurred after the last participant of each cohort had at least completed day 2 assessments.
According to the results of the animal models for cognitive impairment, it was estimated that the effective dose of NX210 varies between 2 and 10 mg/kg. The calculation of the starting dose in humans was based on the current FDA guidelines [11]. Toxicological studies in rats and dogs determined the no-observed-adverse-effect level, and the human equivalent dose selected was the lowest dose (considered equal to 8 mg/kg) after a single dose. As a precaution, a safety factor of 20 was applied. The maximum recommended starting dose was considered equal to 0.4 mg/kg, which was the first dose selected for dose escalation. The range of effective doses in animals corresponded to the mid-end of the dose escalation performed during this first-in-human clinical study.
There was no statistical hypothesis for this study. The sample size calculation was based on empirical considerations.
Participants
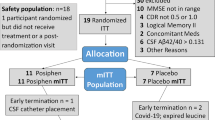
The study involved 39 healthy male and female volunteers aged 18–65 years, with body weight ranging from 50.0 to 90.0 kg and body mass index ranging from 18.0 to 30.0 kg/m2. Participants were enrolled into five cohorts and randomized at a 3:1 ratio to receive NX210 or placebo in five single ascending doses (eight participants per cohort, with the exception of cohort 2 which included seven participants). All participants provided written informed consent prior to their participation in this investigation.
Interventions
Eligibility was assessed during a screening period of up to 4 weeks. Participants were admitted to the clinic on day − 1 and discharged from the clinic on day 2, at least 24 h after dosing. On day 1, participants received a single intravenous bolus injection/infusion of NX210 (0.4, 1.25, 2.5, 5, or 10 mg/kg) or placebo. The duration of administration was less than 12 min. Participants were asked to remain in the supine or semi-recumbent position during the intravenous administration. An equal volume of reconstituted NX210 or corresponding vehicle was administered to all participants of the same cohort. In each cohort, the duration of infusion depended on the drug concentration.
For participants in the 2.5, 5, and 10 mg/kg cohorts undergoing PD assessments, an ambulatory visit took place on day 3. Upon completion of the treatment period, participants returned to the clinic on day 7 (± 1 day) for follow-up assessments. The total duration of the study for each participant was approximately 10 days.
Assessment Parameters
The primary endpoint of this first-in-human study was the safety and tolerability of intravenous administration of single doses of NX210 in healthy volunteers. The following were defined as parameters relevant to safety and tolerability: physical examination; AE and serious AE (SAE); clinical laboratory assessments (e.g., hematology, biochemistry, and urinalysis); 12-lead electrocardiography; telemetric recording; vital signs; and local tolerability at the site of administration.
The medical history, AE, and SAE were coded using Version 23.1 of MedDRA and listed. The treatment-emergent AE (TEAE) were tabulated according to the System Organ Class. Local tolerability (dryness, redness, swelling, pain, and itching) was assessed by the investigator, and any local reaction was rated according to the Common Terminology Criteria for Adverse Events v3.0. Clinically significant local reactions (i.e., grade ≥ 2) had to be reported by the investigator as an AE.
The secondary endpoint was the PK profile induced by NX210 via its metabolite NX210c in plasma up to 180 min after dosing at all dosages. Blood PK samples (4 mL) for the analysis of NX210 via its metabolite NX210c were collected prior to dosing, immediately after bolus injection or termination of the infusion and at 5, 10, 15, 20, 25, 30, 70, 120, and 180 min after bolus injection or termination of infusion. Blood was collected in K2EDTA collection tubes, processed to plasma, and stored at − 80 °C until analysis. Concentrations of NX210c in plasma were quantified using a validated good laboratory practice high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) assay. The quantitation range was 1.50–500 ng/mL. For plasma NX210c samples, the accuracy (bias) for the quality control samples was between − 1.9% and 1.5%, and the precision (% coefficient of variation) for the quality control samples was between 2.5% and 7.2%, respectively.
Exploratory endpoints were assessed in the three cohorts treated with the highest doses and included (1) the PD profile of NX210 by analyzing plasma biomarkers (serotonin, tryptophan, glutamate, glutamine, homocysteine, fibrinogen), urine biomarkers (gamma-aminobutyric acid [GABA], glutamate, adrenaline, noradrenaline, dopamine, serotonin, vanillylmandelic acid, 5-hydroxyindolacetic acid, homovanillic acid), and neurophysiological effects through EEG; and (2) the dose-related effects of NX210.
For these biomarkers, blood was collected before treatment and at 70 min and 10, 24, and 48 h after drug administration. The collection of blood samples was performed under fasting conditions. Participants were not allowed to eat or drink (except water) for a period of 4 h prior to blood sampling. The samples were centrifuged for 10 min at 2000×g. Collected samples were stored at − 70 °C until analysis of biomarkers. HPLC–MS/MS and HPLC were used to determine the levels of amino acids (e.g., tryptophan, homocysteine, glutamate, and glutamine) and serotonin, respectively. Serotonin, glutamate, glutamine, tryptophan, and homocysteine were selected as exploratory blood biomarkers to investigate the pharmacological effects of NX210.
Urine was collected at the same time points as blood for biomarkers (i.e., second morning urine before treatment and at 10, 24, and 48 h after drug administration). The samples were mixed with a stabilizer and stored at − 20 °C until HPLC analysis of biomarkers (neurotransmitters and catabolites). The recorded concentrations of urinary biomarkers were normalized to that of creatinine. For the investigation of the pharmacological effects of NX210, serotonin, glutamate, 5-hydroxyindolacetic, GABA, norepinephrine, epinephrine, dopamine, vanillylmandelic acid, and homovanillic acid were selected as exploratory urinary biomarkers.
For the EEG assessments, five measurements were scheduled per participant. These included one pre-dose and four post-dose measurements (i.e., shortly after administration [40 min], and at 10, 24, and 48 h after dosing). EEG measurements were recorded under two conditions, namely eyes open and eyes closed. In total, 21 active recording electrodes were applied to the scalp in accordance with the International 10–20 System of Electrode Placement. Participants were in the supine or semi-recumbent position in a quiet environment throughout the procedure, which included two resting state sessions, one with eyes open and one with eyes closed, each lasting approximately 10 min. The screening EEG session lasted approximately 25 min.
All analyses and preprocessing at the participant level were performed using the MNE software version 0.21.2 (https://doi.org/10.5281/zenodo.4298746)—aPythonEEG analysis suite developed in 2012 by leading researchers in the field of EEG [12]. The MNE software (https://mne.tools/stable/index.html) [13] was used for signal processing and feature extraction. Subsequent analysis was performed on artifact-free signal segments. Signal quality was ensured using the following three-level approach: (1) visual inspection of recordings for the identification of “bad” channels; (2) detection of eye blinks and other major artifacts through independent component analysis [14]; and (3) automatic rejection of epochs containing artifacts using the auto-reject algorithm [15]. The overall rate of epoch rejection was 10%. The main quantitative endpoint of the EEG analysis was spectral analysis using multi-tappers [16], yielding power spectral densities and power bands. For comparisons between individuals, the power spectra and bands were expressed relatively to the total power. The power spectra and bands were averaged for groups of interest. Moreover, a visual inspection was performed.
Statistical Analysis
The population sets were defined as follows. The all-treated set included all randomized participants who received at least the dose of the study drug. The safety set included participants from the all-treated set who had undergone at least one safety assessment post baseline. The safety set was utilized in the analysis of tolerability and safety variables and corresponded to the all-treated set. The PK set comprised all participants included in the all-treated set who had available samples for PK analysis and did not violate the protocol. The PD data set comprised all participants included in the all-treated set who had available PD samples and did not violate the protocol.
Data on safety/tolerability were analyzed using descriptive statistics. For the blood/urine biomarkers, the mean (± 95% confidence interval) or individual concentrations over time, and the mean (± 95% confidence interval) or individual changes from baseline were determined.
PK data were analyzed according to a dedicated PK statistical analysis plan. The plasma PK parameters of NX210c were calculated using non-compartmental approaches. The PK parameters were computed using Phoenix WinNonlin™, version 6.3 (Certara USA Inc., Princeton, NJ, USA). Actual elapsed sampling times relative to dosing time were used for the estimation of PK parameters.
Owing to the small number (n = 6) of treated participants included in each cohort and the large variability, possible outlier values may affect the mean values considerably. Therefore, geometric means (GM) were also calculated and evaluated for the PK parameters.
A population PK-PD analysis data set was prepared on the basis of clinical data extracted from the cleaned trial databases of the study. The relationship between doses of NX210 and PD effects on blood and urine biomarkers was assessed. Only corresponding relevant results are reported.
EEG assessments were performed only in participants included in the 2.5, 5, and 10 mg/kg cohorts. The EEG power was analyzed for the following frequency bands: alpha, beta, gamma, delta, and theta.
For the main statistical analysis, all participants receiving placebo were combined into one group, irrespective of the cohort that they were assigned to (n = 6). The effect of NX210 dose on the EEG power spectrum was evaluated as a function of time. Detected effects were classified into the alpha, beta, gamma, delta, and theta frequency bands to aid interpretation. A power spectrum was obtained using a Fourier transform. Initially, these power spectra were analyzed through a between-participant approach. Additionally, between-participant and/or between-dose analysis was performed to correct for possible placebo effects. MATLAB (MathWorks, Portola Valley, CA, USA) was employed to perform one-way analysis of variance (ANOVA), using treatment as a grouping variable. Additionally, a mixed effects model was performed using all participants. For all statistical analyses, an alpha of 0.05 (uncorrected) was used as a threshold to denote statistical significance.
Post hoc analyses (time- and dose-dependent) were also conducted because of the small sample size and high interindividual variability. It was decided to group the four relevant EEG channels with availability of reliable measurements for all participants, along the anteroposterior axis (F3, Cz, T4, O2), by averaging them into a single virtual channel. In the time-dependent analysis, data from the 2.5, 5, and 10 mg/kg cohorts were combined. For each time point, relative changes versus the baseline (before treatment) were calculated (i.e., n = 14 for baseline, n = 13 for 40 min, n = 12 for 24 h, and n = 16 for 48 h). In the dose-dependent analysis, 40 min, 24 h, and 48 h data were combined (i.e., n = 14 for placebo, n = 16 for 2.5 mg/kg, n = 14 for 5 mg/kg, and n = 11 for 10 mg/kg). All results are presented as the mean percentage changes ± standard error (SE). For the post hoc analysis, the data were processed using the statsmodels software (https://www.statsmodels.org/stable/index.html#) [17]. For each situation (time or dose effects), one-way ANOVA was performed; p = 0.05 denoted statistical significance.
Results
Participants
One participant (cohort 2; dose 1.25 mg/kg) did not visit the center. Hence, 39 participants (35 male and four female) were enrolled in this study. Of those, the vast majority were White (n = 35; 89.7%); the remaining four participants were Asian (n = 3; 7.7%) and Black or African American (n = 1; 2.6%). The mean age of the participants was 41.9 years, and the mean body mass index was 24.1 kg/m2. Of note, one participant from cohort 5 (10 mg/kg) violated the protocol (i.e., receiving treatment with tamsulosin for bladder urothelial carcinoma prior to screening) and was not included in the PK and PD analyses (the participant did not declare the carcinoma at the eligibility assessment; this information was provided after enrolment by his general practitioner). The characteristics of the participants are shown in Table 1. The dosing regimens for the five cohorts are provided below:
-
Cohort 1: 0.4 mg/kg NX210 (n = 6) or placebo (n = 2) by bolus injection
-
Cohort 2: 1.25 mg/kg NX210 (n = 5) or placebo (n = 2) by 2-min bolus injection
-
Cohort 3: 2.5 mg/kg NX210 (n = 6) or placebo (n = 2) by 4-min infusion
-
Cohort 4: 5 mg/kg NX210 (n = 6) or placebo (n = 2) by 7-min infusion
-
Cohort 5: 10 mg/kg NX210 (n = 6) or placebo (n = 2) by 12-min infusion
Thus, a total of 10 and 29 participants received placebo and NX210, respectively.
Safety and Tolerability
A total of 17 TEAE were recorded in 13 participants (33%). These included 12 events (70.6%) in nine participants (23%), which were deemed related to the study drug. Six among those 12 events concerned the 1.25 mg/kg dose. Of the related TEAE, seven events (58.3%) concerned nervous system disorders (e.g., dizziness, headache, somnolence), among which four concerned the 1.25 mg/kg dose. TEAE related to the study drug and reported by more than 5% of the participants included dizziness (three events), headache (one event), and somnolence (two events). Other related TEAE concerned the gastrointestinal system (one event), cardiac disorders (one event), eye disorders (one event), renal disorders (one event), and general disorders (one event).
All TEAE were of mild severity and resolved spontaneously, except for one (worsening of leukocyturia) with unknown outcome. The treatment-related AE are summarized in Tables 2, 3, and 4.
The onset of four (23.5%) and two (11.7%) TEAE occurred 0–30 and 60–120 min after administration, respectively. Most TEAE (11 events; 64.7%) developed more than 120 min following administration. Overall, there were no dose- or time-dependent changes observed in any of the vital signs, physical examinations, clinical chemistry, telemetric recordings, or electrocardiography parameters.
Regarding tolerability, four participants (10%) reported nine tolerability issues of mild intensity (e.g., redness, swelling, pain at the administration site). Only one participant in the 10 mg/kg cohort reported moderate (grade 2) pain (blood collection catheter site-related reaction) 24 h post treatment.
According to these results, NX210 (administered once through the intravenous route) showed a good safety and tolerability profile, irrespective of the dose.
Pharmacokinetics
The analysis showed that the GM of the maximum concentration (GM, Cmax) is increased with the dose (299, 405, and 485 ng/mL for 2.5, 5, and 10 mg/kg dose cohort, respectively). The same observation was noted for GM area under the curves (AUC) last (1080, 1710, and 3960 min·ng/mL for the 2.5, 5, and 10 mg/kg doses, respectively). NX210c was rapidly eliminated, with the average plasma half-life values for the three highest doses (i.e., 2.5, 5, and 10 mg/kg) ranging from 6.19 to 20.0 min. At 60 min after administration, NX210c was no longer detected in the plasma (Fig. 1). The volume of distribution and total clearance ranged from 1870 to 4120 L and from 7440 to 16,400 L/h, respectively, indicating rapid clearance and high tissue distribution. The time to maximum (Tmax) of the 2.5 mg/kg dose (4 min) was equivalent to the duration of infusion, while that of the 5 and 10 mg/kg doses (4 and 10 min, respectively) was shorter (7 and 12 min, respectively) (Table 5). Dose proportionality could not be determined because of the high variability in the PK results and the small sample size.
Pharmacodynamics (Exploratory) Analysis
All plasma and urine biomarkers concentrations were above the lower limit of quantification.
Plasma Biomarkers
Dose-related effects were observed for homocysteine and tryptophan (Fig. 2). In comparison with placebo, the mean percentage changes from baseline indicated an increase and a decrease in the levels of tryptophan (up to 55.1% [SE = 7.42]) and homocysteine (up to 19.6% [SE = 1.88]), respectively, 70 min after the administration of NX210 at the concentration of 10 mg/kg. Also, an exploratory population PK-PD model [18] detected dose-related variations for both homocysteine and tryptophan plasma biomarkers (data not shown).
At 10 h, changes from baseline showed variations without a dose effect for plasma glutamine and glutamate in the treatment groups versus the placebo group (increase and decrease, respectively; data not shown).
Urinary Biomarkers
For the dose of 10 mg/kg, the mean percentage changes from baseline over time showed an increasing trend in the urinary excretion of serotonin at 70 min and 48 h (mean = 55.2% [SE = 33.0] and 19.0% [18.9], respectively (Fig. 3); glutamate at 10 h (mean = 57.5% [SE = 37.3]); and GABA at 10 and 48 h (mean = 74.7% [SE = 45.1] and 30.0% [SE = 19.9], respectively (data not shown). This was particularly notable in participants in cohorts 4 and 5 versus those who received placebo. Moreover, the PD urinary variations were sustained, lasting for up to 48 h after the administration of NX210, particularly for serotonin and GABA.
However, on the basis of the analysis of urine biomarkers, there was no evidence of a marked drug dose-related effect (data not shown).
EEG Results
The results showed that the power was within specific frequency bands (beta [12–30 Hz] and gamma [30–80 Hz]) compared with placebo, as a result of the treatment. More precisely, a statistically significant effect was found for the beta bands during the eyes open evaluation 24 h after the administration of NX210 (p = 0.042). A statistically significant effect for the gamma bands was observed during the eyes closed examination 40 min (p = 0.049) and 48 h (p = 0.016) after the administration of NX210. In all these cases, a statistically significant reduction in power was observed (Fig. 4).
For the post hoc analyses, the EEG activity (with all doses combined) after a single intravenous administration of NX210 over time showed a trend for increasing variation in the alpha power bands (p = 0.84), with a maximum observed at 40 min (17.5% [SE = 16.9]); this variation was sustained at 24 h and 48 h (14.3% [SE = 19.5] and 13.2% [SE = 13.1], respectively) (Fig. 5).
As shown in Fig. 5, the variations in alpha bands (with all time points combined) appeared to be dose-related; however, they were not statistically significant (p = 0.75). The mean percentage changes for the placebo, and 2.5, 5, and 10 mg/kg groups were 4.8% (SE = 10.6), 9.5% (SE = 11.6), 10.7% (SE = 17.9), and 28.2% (SE = 19.7), respectively.
Discussion
In the present study, the single intravenous administration of NX210 showed a very good safety and tolerability profile, irrespective of the dose.
These findings are consistent with the general concept that peptides are well-tolerated drugs with good safety profiles compared with small molecules, antibodies, and biologics [19]. Short peptides are characterized by numerous advantages, such as a good safety profile, low toxicity, and low immunogenicity [20]. Moreover, the good safety profile of a single injection or short infusion of NX210 has been demonstrated in animal toxicological, PK, and preclinical safety studies. In vitro studies revealed that NX210 is not cardiotoxic, mutagenic, or cytotoxic (unpublished data).
The observed greater number of AE reported in the 1.25 mg dose group is not related to the Cmax that was very low compared with that noted in the 5 mg dose group (43.5 vs. 461 ng/mL, respectively). This indicates that the occurrence of the related AE was not linked to the Cmax. Moreover, the severity of related AE was not greater with the increase in dose. Considering that this was a single ascending dose first-in-human study, these results are exploratory. Further research will be conducted to understand this observation, in particular following repeated injections of NX210.
The PK analysis showed that the Cmax and AUC (GM) increased with the dose. The NX210c was rapidly eliminated and no longer detectable in the plasma 60 min after administration, denoting a short-term exposure. The short half-life of NX210c found in the current study (6–20 min) was similar to those previously observed in three animal species used for single-dose PK evaluation (i.e., 12 min in primates, 12 min in rats, and 24 min in dogs). Regardless of the dose, sex, and animal species, NX210c was not detected between 1 and 3 h after dosing (unpublished data). In general, peptides have a relatively short half-life [20] and rapid clearance due to their susceptibility to rapid digestion by proteolytic enzymes (proteases/peptidases) in the systemic circulation. In this study, high volume of distribution and high total clearance were observed, indicating rapid clearance and high tissue diffusion.
The high tissue affinity and diffusion may be explained by the high binding affinity of peptides for a wide range of targets. Moreover, the Cmax in the three highest dose groups was recorded at the same time or even before the end of the infusion, which may be explained by rapid binding and high tissue affinity. Overall, the PK profile of NX210c suggests the presence of more than one distribution compartment.
The blood and urine biomarkers examined in this first-in-human study in healthy volunteers are common neurotransmitters or amino acids (i.e., GABA, glutamate, and serotonin in urine; serotonin, tryptophan, glutamate, glutamine, and homocysteine in blood). Neurotransmitters play a central role in brain function; hence, neurotransmitter receptors are critical targets in the development of therapeutics for the treatment of psychiatric and neurological disorders [21]. Glutamate is not specific for neurodegenerative diseases. Nevertheless, it is the most prominent neurotransmitter in the brain, where it is both the main excitatory (in its glutamate form) and the main inhibitory component (as precursor of GABA). Glutamate is crucial in neural communication, as it is involved in the synaptic current mediated by ion channels. Moreover, for many years, it has been hypothesized that glutamatergic dysfunction and neuronal overstimulation (i.e., excitotoxicity) are involved in the mechanisms underlying neuronal loss in neurodegenerative diseases. It has been demonstrated that NX210 prevents glutamate-related cell death (i.e., excitotoxicity) in many different primary neuronal cell types of human or rodent origin, such as dopaminergic, GABAergic, hippocampal, cortical, and motor neurons [10].
The levels of neurotransmitters in the brain can be determined through CSF analysis. However, since this was a first-in-human study in healthy volunteers rather than a study in the target population (e.g., with cognitive impairment), the invasive methodology of continuous CSF sampling could not be justified. Data have shown positive correlations between the levels of biomarkers in the blood and CSF, particularly for glutamate and homocysteine [22,23,24]. Of note, this correlation was weaker for urine biomarkers.
The PD urine analysis revealed large interindividual variability for all investigated biomarkers. Although dose-related variation was not observed, increased urinary excretion of the neurotransmitters serotonin, GABA, and glutamate was observed following the intravenous administration of NX210 (particularly at the highest doses: 5 and 10 mg/kg). These findings suggest that NX210, potentially via its metabolite NX210c, may exert an effect on cognitive functions.
In the plasma, the levels of tryptophan and homocysteine showed variations with a dose-related drug effect (i.e., increase and decrease, respectively).
Tryptophan is an essential amino acid precursor for a number of metabolites, most notably kynurenine and serotonin. Both are involved in behavioral and cognitive symptoms of neurological diseases [25]. Kynurenine is the precursor of kynurenic acid, an antagonist of glutamate ionotropic receptors. Serotonin is a neurotransmitter linked to the modulation of a wide array of functions, including sleep, control of appetite and temperature, mood, and cognition [25]. In the 1970s, Yuwiler et al. [26] showed that the production of brain serotonin is dependent on the amount of circulating tryptophan. Widner et al. [27] found that decreased tryptophan levels and increased kynurenine levels in the serum correlated with the degree of cognitive decline in patients with AD. More recently, Ramos-Chávez et al. [28] demonstrated for the first time that tryptophan metabolites are correlated with cognitive performance during normal aging in women. They found a negative correlation between age and tryptophan levels, as well as a positive influence of age on the kynurenine/tryptophan ratio. A strong relationship between activation of the kynurenine pathway and cognitive impairment was also observed in AD, which is an age-related neurodegenerative disease. The investigators concluded that the levels of tryptophan may be a useful indicator of cognitive impairment in women aged over 50 years [28]. However, it is important to note that the dose-related effect observed for tryptophan in our study may be partly attributed to the degradation of NX210 (that contains two tryptophans in its sequence).
In contrast, the results obtained for homocysteine may reflect one of the pharmacological effects of the NX210 on related cognition function. Homocysteine is a sulfur-containing amino acid derived from methionine metabolism. Elevated plasma levels of homocysteine play a causal role in several neurological conditions [23, 24, 29, 30]. In the last 2 decades, the role of homocysteine as a risk factor for CNS diseases has attracted considerable attention. Recently, two meta-analyses reported that increased plasma levels of homocysteine were correlated with increased risk for all types of dementia, including AD [31, 32]. In 2021, using data from almost 7500 patients, Zuin et al. linked higher levels of homocysteine to an increase in the risk of developing AD [31]. The investigators concluded that homocysteine may play an important role in AD-related neurodegeneration. They also stated that, in elderly individuals, higher blood levels of homocysteine are associated with a greater risk of developing AD. Also, Wang et al. [32] reported a pooled overall risk ratio of 1.22 (95% confidence interval 1.08–1.36) for all types of dementia. It was estimated that an increase in the plasma levels of homocysteine by 5 µmol/L was associated with a 9% and 12% increase in the risk of developing dementia and AD, respectively [32]. The neurotoxic mechanisms of homocysteine have also been associated with post-viral infection conditions. Neurological disturbances (including cognitive impairment and anosmia) are common manifestations in patients with COVID-19 [33]. On the basis of the established role of homocysteine in inflammatory processes, Ponti et al. concluded that genetic data related to the status of the methylenetetrahydrofolate reductase gene coupled with the plasma levels of homocysteine could represent important information for the assessment and stratification of patients with acute COVID-19 infection [33]. Hence, according to the authors, a preventive therapeutic approach by decreasing the plasma levels of homocysteine may reduce the severity and mortality associated with COVID-19.
The pathogenesis of homocysteine-induced vascular and CNS damage may be multifactorial, including direct damage to the endothelium, stimulation of smooth muscle cell proliferation, enhanced peroxidation of low-density lipoprotein, increase in platelet aggregation, and effects on the coagulation system [34].
In clinical trials, a 25–30% decrease in the plasma concentration of homocysteine has been associated with improved cognitive outcomes [35]. Therefore, the rapid reduction in the levels of homocysteine (19.6%) observed in the present phase 1 study with healthy volunteers following a single intravenous administration of NX210 at 10 mg/kg is a promising finding. We hypothesized that the peptide could bind with homocysteine and, hence, improve its elimination. This hypothesis was supported by in silico modeling and docking simulations, especially due to the two cysteines contained in the NX210c sequence (unpublished data). However further exploration is warranted to elucidate and/or confirm the mechanism involved in the systemic reduction of homocysteine by NX210/NX210c in a larger sample with repeated injections.
Overall, after a single intravenous administration, NX210 exerted a marked dose-related effect. This effect resulted in blood PD variations (plasma tryptophan and homocysteine) lasting for at least 10 h, with the maximum effect observed at 70 min. NX210c is not quantifiable after 60 min in blood. Therefore, the observed PD effects are sustained longer than the quantifiable exposure of the NX210c in blood.
Besides tryptophan and homocysteine, the PD analysis showed increasing and decreasing variations for the plasma levels of glutamine and glutamate, respectively, at 10 h in participants treated with NX210/NX210c (data not shown). Recently, studies demonstrated the neuroprotective action of NX210c against glutamate-induced excitotoxicity in primary rat cortical and hippocampal neurons, as well as human cortical neurons [9, 10]. Interestingly, glutamate (a major excitatory neurotransmitter) is highly abundant throughout the brain; it plays a crucial role in neuronal plasticity and the maintenance of cognitive processing. In humans, the levels of glutamate measured in the blood are positively correlated with those detected in the CSF [22]. The glutamate–glutamine cycle is critical for maintaining synaptic function and impaired in various disorders of the CNS [36]. In this study, a clear drug dose-related effect was not found for the plasma glutamate or glutamine biomarkers. Also, there was large interindividual variability due to the limited number of participants. Nevertheless, these findings suggest that NX210/NX210c may play a role in the glutamate–glutamine cycle.
Van Straaten et al. showed EEG changes in the course of AD, including the slowing of EEG oscillations, decreased functional connectivity in higher-frequency bands, and decline in functional network organization. They concluded that EEG is a feasible and potentially useful addition to cognitive endpoint measures in clinical trials of AD [37]. More recently, Stoiljkovic et al. reported that neurophysiological signals could be applied to drug discovery efforts for the development of therapies with disease-modifying potential [38].
The effects of cognition-enhancing agents on beta and gamma band activity remain controversial. In a recent article on the use of gamma oscillations for drug development, Honda et al. indicated that baseline gamma power is increased in animal models of neuropsychiatric disorder created by blunting N-methyl-d-aspartate signaling transduction in parvalbumin-positive interneurons [39]. Thus, reduction of baseline power may be a commendable goal for therapeutic interventions. A trial investigating noninvasive brain stimulation of the frontal cortex reported a decrease in resting power in the beta and gamma bands [40]. This supports the notion of reduced beta and gamma power after a cognitive-enhancing intervention in healthy participants.
In patients with AD, typical features of EEG include increased delta or theta rhythm and decreased alpha or beta rhythm activities [41]. In the current analysis, although non-statistically significant, the results showed a dose-dependent increase in alpha bands. Recently, Meghdadi et al. explored the utility of resting-state EEG measures as potential biomarkers for the detection and assessment of cognitive decline in mild cognitive impairment and AD. They concluded that changes in slower frequencies in AD are specific to pathologic cognitive decline in patients with AD [42]. Hence, an increase in alpha frequency induced by NX210/NX210c may exert a beneficial effect in this setting. Previous results showed that the amplitude of alpha 1 and 2 sources in occipital, temporal, and limbic areas was significantly lower in individuals carrying the epsilon4 allele than in those without this allele in both mild cognitive impairment and AD [43]. In a recent review, Babiloni et al. highlighted that treatment with acetylcholinesterase inhibitors (e.g., donepezil) results in an increase in dominant alpha rhythms, as well as improvement in the cognitive status of patients with AD [44]. The present study yielded some preliminary evidence evocative of a similar effect of NX210/NX210c on the CNS. This effect is probably exerted by direct or indirect enhancement of the thalamo-cortical and/or cortico-cortical interactions that drive the alpha EEG activity, as previously described [45, 46]. This finding could broaden the field of possible clinical applications of NX210/NX210c to other neurological diseases characterized by a decreased alpha power band, such as PaD and/or Lewy body dementia [47,48,49,50]. Moreover, it supports a pharmacological effect of the therapeutic peptide, regardless of a putative interaction with plasma homocysteine.
The limitations of the present investigation should be acknowledged. Firstly, this was an exploratory study with a small sample size and high interindividual variability. Therefore, as a result of the low number of participants per treatment group, the observed alterations in blood and urine biomarkers should be interpreted with caution. Secondly, the majority of data in this study were descriptively analyzed (except for those of EEG). Finally, exploratory biomarkers were limited to general neurotransmitters, not specific to a particular neurodegenerative disease.
Conclusion
In this first-in-human single ascending dose phase 1 clinical trial, a single intravenous administration of NX210 in adult healthy volunteers showed a good safety and tolerability profile, regardless of the dose.
Considering the PD results, the present analysis provides preliminary evidence concerning the effect of NX210 and its metabolite NX210c on the CNS, in particular on cognitive processing. While NX210c was rapidly eliminated in the systemic circulation, the PD variations measured were sustained (several hours in plasma, up to 48 h in urine and EEG).
Further preclinical assessments to fully elucidate the mechanism of action of NX210c, as well as additional clinical investigations with larger sample sizes and repeated administrations, are warranted to assess the effectiveness of direct administration of NX210c. However, by combining multiple properties in a single drug, NX210c may represent a new pharmacological treatment option for neurocognitive diseases (e.g., AD or post-viral infection conditions).
References
Patterson C. World Alzheimer Report 2018. London: Alzheimer’s Disease International 2018. Published 21 September 2021. https://www.alzint.org/u/WorldAlzheimerReport2018.pdf. Accessed 29 May 2022.
Shen WB, Logue J, Yang P, et al. SARS-CoV-2 invades cognitive centers of the brain and induces Alzheimer’s-like neuropathology. bioRxiv. 2022. https://doi.org/10.1101/2022.01.31.478476.
Boutajangout A, Frontera J, Debure L, Vedvyas A, Faustin A, Wisniewski T. Plasma biomarkers of neurodegeneration and neuroinflammation in hospitalized COVID-19 patients with and without new neurological symptoms. Alzheimer's Dement. 2021;17(Suppl. 5):e057892. https://doi.org/10.1002/alz.057892.
Weller J, Budson A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Res. 2018;12:49. https://doi.org/10.12688/f1000research.14506.1.
Sachdev PS, Lo JW, Crawford JD, et al. STROKOG (stroke and cognition consortium): an international consortium to examine the epidemiology, diagnosis, and treatment of neurocognitive disorders in relation to cerebrovascular disease. Alzheimers Dement (Amst). 2016;7:11–23. https://doi.org/10.1016/j.dadm.2016.10.006.
GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:8–106. https://doi.org/10.1016/S1474-4422(18)30403-4.
Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7:47. https://doi.org/10.1038/s41572-021-00280-3.
Sakka L, Delétage N, Lalloué F, et al. SCO-spondin derived peptide NX210 induces neuroprotection in vitro and promotes fiber regrowth and functional recovery after spinal cord injury. PLoS One. 2014;9: e93179. https://doi.org/10.1371/journal.pone.0093179.
Le Douce J, Delétage N, Bourdès V, Lemarchant S, Godfrin Y. Subcommissural organ-spondin-derived peptide restores memory in a mouse model of Alzheimer’s disease. Front Neurosci. 2021;15: 651094. https://doi.org/10.3389/fnins.2021.651094.
Delétage N, Le Douce J, Callizot N, Godfrin Y, Lemarchant S. SCO-spondin-derived peptide protects neurons from glutamate-induced excitotoxicity. Neuroscience. 2021;463:317–36. https://doi.org/10.1016/j.neuroscience.2021.02.005.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry—Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Published July 2005. https://www.fda.gov/media/72309/download. Accessed 29 May 2022.
Gramfort A, Luessi M, Larson E, et al. MNE software for processing MEG and EEG data. Neuroimage. 2014;86:446–60. https://doi.org/10.1016/j.neuroimage.2013.10.027.
Gramfort A, Luessi M, Larson E, et al. MEG and EEG data analysis with MNE-Python. Front Neurosci. 2013;7:267. https://doi.org/10.3389/fnins.2013.00267.
Ablin P, Cardoso J, Gramfort A. Faster independent component analysis by preconditioning with Hessian approximations. IEEE Trans Signal Process. 2018;66:4040–9. https://doi.org/10.1109/TSP.2018.2844203.
Jas M, Engemann DA, Bekhti Y, Raimondo F, Gramfort A. Autoreject: automated artifact rejection for MEG and EEG data. Neuroimage. 2017;159:417–29. https://doi.org/10.1016/j.neuroimage.2017.06.030.
Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE. 1982;70:1055–96. https://doi.org/10.1109/PROC.1982.12433.
Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. In: Proceedings of the 9th Python in Science Conference; 2010. pp. 92–6. https://doi.org/10.25080/MAJORA-92BF1922-011.
Sheiner LB, Steimer JL. Pharmacokinetic/pharmacodynamic modeling in drug development. Annu Rev Pharmacol Toxicol. 2000;40:67–95. https://doi.org/10.1146/annurev.pharmtox.40.1.67.
Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–8. https://doi.org/10.1016/j.drudis.2014.10.003.
Apostolopoulos V, Bojarska J, Chai TT, et al. A global review on short peptides: frontiers and perspectives. Molecules. 2021;26:430. https://doi.org/10.3390/molecules26020430.
Hyman SE. Neurotransmitters. Curr Biol. 2005;15(5):R154–8. https://doi.org/10.1016/j.cub.2005.02.037.
Alfredsson G, Wiesel FA, Tylec A. Relationships between glutamate and monoamine metabolites in cerebrospinal fluid and serum in healthy volunteers. Biol Psychiatry. 1988;23:689–97. https://doi.org/10.1016/0006-3223(88)90052-2.
Selley ML, Close DR, Stern SE. The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer’s disease. Neurobiol Aging. 2002;23:383–8. https://doi.org/10.1016/s0197-4580(01)00327-x.
Smach MA, Jacob N, Golmard JL, et al. Folate and homocysteine in the cerebrospinal fluid of patients with Alzheimer’s disease or dementia: a case control study. Eur Neurol. 2011;65:270–8. https://doi.org/10.1159/000326301.
Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8:56. https://doi.org/10.3390/nu8010056.
Yuwiler A, Oldendorf WH, Geller E, Braun L. Effect of albumin binding and amino acid competition on tryptophan uptake into brain. J Neurochem. 1977;28:1015–23. https://doi.org/10.1111/j.1471-4159.1977.tb10664.x.
Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D. Tryptophan degradation and immune activation in Alzheimer’s disease. J Neural Transm (Vienna). 2000;107:343–53. https://doi.org/10.1007/s007020050029.
Ramos-Chávez LA, Roldán-Roldán G, García-Juárez B, et al. Low serum tryptophan levels as an indicator of global cognitive performance in nondemented women over 50 years of age. Oxid Med Cell Longev. 2018;2018:8604718. https://doi.org/10.1155/2018/8604718.
Valentino F, Bivona G, Butera D, et al. Elevated cerebrospinal fluid and plasma homocysteine levels in ALS. Eur J Neurol. 2010;17:84–9. https://doi.org/10.1111/j.1468-1331.2009.02752.x.
Ahlgren E, Hagberg L, Fuchs D, et al. Association between plasma homocysteine levels and neuronal injury in HIV infection. PLoS One. 2016;11:e0158973. https://doi.org/10.1371/journal.pone.0158973.
Zuin M, Cervellati C, Brombo G, Trentini A, Roncon L, Zuliani G. Elevated blood homocysteine and risk of Alzheimer’s dementia: an updated systematic review and meta-analysis based on prospective studies. J Prev Alzheimers Dis. 2021;8:329–34. https://doi.org/10.14283/jpad.2021.7.
Wang Q, Zhao J, Chang H, Liu X, Zhu R. Homocysteine and folic acid: risk factors for Alzheimer’s disease-an updated meta-analysis. Front Aging Neurosci. 2021;13: 665114. https://doi.org/10.3389/fnagi.2021.665114.
Ponti G, Pastorino L, Manfredini M, et al. COVID-19 spreading across world correlates with C677T allele of the methylenetetrahydrofolate reductase (MTHFR) gene prevalence. J Clin Lab Anal. 2021;35: e23798. https://doi.org/10.1002/jcla.23798.
Fridman O. Hiperhomocist(e)inemia: aterotrombosis y neurotoxicidad [Hyperhomocysteinemia: atherothrombosis and neurotoxicity]. Acta Physiol Pharmacol Ther Latinoam. 1999;49:21–30 (Spanish).
Smith AD, Refsum H, Bottiglieri T, et al. Homocysteine and dementia: an international consensus statement. J Alzheimers Dis. 2018;62:561–70. https://doi.org/10.3233/JAD-171042.
Shimmura C, Suda S, Tsuchiya KJ, et al. Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS One. 2011;6:e25340. https://doi.org/10.1371/journal.pone.0025340.
van Straaten EC, Scheltens P, Gouw AA, Stam CJ. Eyes-closed task-free electroencephalography in clinical trials for Alzheimer’s disease: an emerging method based upon brain dynamics. Alzheimers Res Ther. 2014;6:86. https://doi.org/10.1186/s13195-014-0086-x.
Stoiljkovic M, Kelley C, Horvath TL, Hajós M. Neurophysiological signals as predictive translational biomarkers for Alzheimer’s disease treatment: effects of donepezil on neuronal network oscillations in TgF344-AD rats. Alzheimers Res Ther. 2018;10:105. https://doi.org/10.1186/s13195-018-0433-4.
Honda S, Matsumoto M, Tajinda K, Mihara T. Enhancing clinical trials through synergistic gamma power analysis. Front Psychiatry. 2020;11:537. https://doi.org/10.3389/fpsyt.2020.00537.
Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE. Effects of prefrontal bipolar and high-definition transcranial direct current stimulation on cortical reactivity and working memory in healthy adults. Neuroimage. 2017;152:142–57. https://doi.org/10.1016/j.neuroimage.2017.03.001.
Wang J, Fang Y, Wang X, Yang H, Yu X, Wang H. Enhanced gamma activity and cross-frequency interaction of resting-state electroencephalographic oscillations in patients with Alzheimer’s disease. Front Aging Neurosci. 2017;9:243. https://doi.org/10.3389/fnagi.2017.00243 (eCollection 2017).
Meghdadi AH, Stevanović Karić M, McConnell M, et al. Resting state EEG biomarkers of cognitive decline associated with Alzheimer’s disease and mild cognitive impairment. PLoS One. 2021;16: e0244180. https://doi.org/10.1371/journal.pone.0244180.
Babiloni C, Benussi L, Binetti G, et al. Apolipoprotein E and alpha brain rhythms in mild cognitive impairment: a multicentric electroencephalogram study. Ann Neurol. 2006;59:323–34. https://doi.org/10.1002/ana.20724.
Babiloni C, Del Percio C, Bordet R, et al. Effects of acetylcholinesterase inhibitors and memantine on resting-state electroencephalographic rhythms in Alzheimer’s disease patients. Clin Neurophysiol. 2013;124:837–50. https://doi.org/10.1016/j.clinph.2012.09.017.
Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. https://doi.org/10.1152/physrev.1988.68.3.649.
Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–57. https://doi.org/10.1016/s1388-2457(99)00141-8.
Babiloni C, De Pandis MF, Vecchio F, et al. Cortical sources of resting state electroencephalographic rhythms in Parkinson’s disease related dementia and Alzheimer’s disease. Clin Neurophysiol. 2011;122:2355–64. https://doi.org/10.1016/j.clinph.2011.03.029.
Andersson M, Hansson O, Minthon L, Rosén I, Londos E. Electroencephalogram variability in dementia with Lewy bodies, Alzheimer’s disease and controls. Dement Geriatr Cogn Disord. 2008;26:284–90. https://doi.org/10.1159/000160962.
Polverino P, Ajčević M, Catalan M, Mazzon G, Bertolotti C, Manganotti P. Brain oscillatory patterns in mild cognitive impairment due to Alzheimer’s and Parkinson’s disease: an exploratory high-density EEG study. Clin Neurophysiol. 2022;138:1–8. https://doi.org/10.1016/j.clinph.2022.01.136.
Massa F, Meli R, Grazzini M, et al. Utility of quantitative EEG in early Lewy body disease. Parkinsonism Relat Disord. 2020;75:70–5. https://doi.org/10.1016/j.parkreldis.2020.05.007.
Acknowledgements
The authors thank the participants of the study.
Funding
There was no grant received for this study. Axoltis Pharma was involved in the study design, interpretation of data, and review/approval of the manuscript. QPS Netherlands B.V. was involved in the design and conduct of the study, and collection, analysis, and interpretation of data (in particular PK data). University Medical Center Groningen was involved in the recording, assessment methods, and clinical interpretation of EEGs. Athena Bio Consulting was involved in the analysis and interpretation of data obtained from the EEG analysis. Certara was involved in the PK-PD analyses and related data interpretation. The Rapid Service Fee was provided by Axoltis Pharma.
Editorial and Other Assistance
Special thanks are extended to the members of the Safety Review Committee, Khalid Abd-Elaziz (Principal Investigator, QPS Netherlands B.V.), Wytze Aalders (Co-investigator, QPS Netherlands B.V.), and David Liens (Medical Monitor, BPLS Consulting). The authors wish to thank Khaled Benkali (Director Clinical Pharmacology, Certara) and Philippe Pierrillas (Associate Director, Modeler Consultant, Certara) for their contribution to the PD data analysis and interpretation, as well as Remco Renken (MR Physicist, University Medical Center Groningen) and Jan-Bernard Marsman (Senior Researcher, University Medical Center Groningen) for their contribution to EEG data collection and interpretation. For the blood amino acid and serotonin analysis, the authors thank Dr Cécile Acquaviva (LBMMS, UM Pathologies Héréditaires du Métabolisme et du Globule Rouge) and Dr Laurence Chardon (LBMMS, UM Pathologies Cancéreuses). For the urine PD analysis, the authors thank Laboratoires Réunis, G.-D. Luxembourg. The authors also thank Luca Bolliger (Founder, abcDNA; Chief Business Officer, Axoltis Pharma) for his review of the manuscript. Sotirios Georgantopoulos (SG Medical Writing B.V.) provided editorial assistance, which was funded by Axoltis Pharma.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have approved the final version of this manuscript for publication.
Author Contributions
Conception of the study: Yann Godfrin, Sébastien Marie, Peter Dogterom. Study investigator: Khalid Abd-Elaziz. Safety results: Valérie Bourdès, Sébastien Marie, Khalid Abd-Elaziz. Analysis and interpretation of PK results: Peter Dogterom, Khalid Abd-Elaziz, Thomas Chou, Yann Godfrin, Sébastien Marie, Valérie Bourdès. Analysis and interpretation of blood and urine PD results: Yann Godfrin, Sébastien Marie, Valérie Bourdès. Analysis and interpretation of EEGs: André Aleman (main analysis), Damien Colas, Pierre Parmantier (post hoc analysis). Writing of manuscript: Valérie Bourdès. Review manuscript: Valérie Bourdès, Yann Godfrin, Sébastien Marie, Sighild Lemarchant, Peter Dogterom, Khalid Abd-Elaziz, Thomas Chou, André Aleman, Damien Colas, Pierre Parmantier.
Disclosures
Valérie Bourdès (M.D., MPH, Chief Medical Officer), Sébastien Marie (Clinical Operations Director), and Sighild Lemarchant (Head of Preclinical R&D) are employees of Axoltis Pharma. Yann Godfrin is the Chief Executive Officer and shareholder of Axoltis Pharma. Peter Dogterom (Clinical Pharmacology and Pharmacokinetics Expert) and Khalid Abd-Elaziz (Principal Investigator) are employees of QPS Netherlands B.V. Thomas Chou (Clinical Pharmacokineticist) is an employee of QPS, LLC. André Aleman is a Professor of Cognitive Neuropsychiatry at the University Medical Centre Groningen, Groningen, Netherlands. Pierre Parmantier and Damien Colas are owner and consultant of Athena Bio Consulting, respectively.
Compliance with Ethics Guidelines
The clinical trial protocol, the participant information, and informed consent forms were approved by the Independent Ethics Committee of the trial center (Medisch Ethische Toetsingscommissie Assen) on 12 May 2020 (CCMO code NL73571.056.20; Study code 190166-CS0332). The principles of informed consent were implemented according to the latest version of the Declaration of Helsinki, the International Conference on Harmonization Guideline for Good Clinical Practice, and regulatory requirements (Wet medisch-wetenschappelijk onderzoek met mensen, Nederland).
Data Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bourdès, V., Dogterom, P., Aleman, A. et al. Safety, Tolerability, Pharmacokinetics and Initial Pharmacodynamics of a Subcommissural Organ-Spondin-Derived Peptide: A Randomized, Placebo-Controlled, Double-Blind, Single Ascending Dose First-in-Human Study. Neurol Ther 11, 1353–1374 (2022). https://doi.org/10.1007/s40120-022-00380-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00380-6