Abstract
In this work, an anodic stripping voltammetry for nanomolar determination of diltiazem with a chemically modified carbon paste electrode (CMCPE) containing Co3O4/SnO2 nanopowders was studied. The accumulation potential and time were selected at, −0.2 V and 190 s, respectively. The electroanalytical performance of the CMCPE was evaluated regarding the carbon paste composition, the solution pH, the time and potential accumulation, and the potential interferences. The novel electrode showed linear response to diltiazem concentration range of 50–650 nM with a lowest detection limit value of 15 nM. The precisions for six consecutive determinations of 350 and 550 nM diltiazem were 3.2 and 2.5 %, respectively. It was demonstrated that the proposed method was free from most interference. Finally, the method was effectively applied to the determination of diltiazem in pharmaceutical tablets and biological samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diltiazem, (+5)-5-[2-(dimethylamino) ethyl]-cis,2,3-dihydro-3-hydroxy-2-(p-methoxyphenyl-1,5-benzothiazepin-4(5H)-one acetate monohydrochloride (Fig. 1), is an important coronary vasodilator drug of the calcium channel-blocker, used in the therapy of heart disease and hypertension [1]. The drug has a mean biological half-life of 3–6 h in plasma [2]. The absolute bioavailability of the tablets ranges between 30 and 42 % largely due to presystemic hepatic metabolism [3, 4]. After absorption, diltiazem undergoes extensive hepatic metabolism through three major metabolic pathways, N-demethylation, O-deacetylation, and O-demethylation [2–5]. Diltiazem undergoes hydrolysis to desacetyl diltiazem in acidic solutions and is most stable at pH 5. The extrapolated room temperate shelf-life was 42 or 15.8 days at pH 5 or 2, respectively [6], as well as a stock solution of the drug was prepared daily during the measurements and protect from the light. Its therapeutic and toxic effects require very sensitive methods for determination of trace levels. Various analytical methods have been employed for this purpose, e.g. high-performance liquid chromatography in human cardiac tissue [7], in blood [8] and in human plasma [9–13], high-performance thin-layer chromatographic [14], gas chromatography [15], spectrophotometry [16, 17], liquid–solid extraction [18] and voltammetry [19]. Since employed techniques for determination of diltiazem in the presence of other drugs are mostly spectroscopy and HPLC, and these methods often are complex and consuming cost solvents, must easy-handled and simple-operating methods need be applied.
Recently, we used modified CPEs for trace analysis of some drugs and heavy metals in various environments [20–24]. Newly we showed that the application of nanopowders of Fe2TiO5 in CPEs catalyses the oxidation of salbutamol and enhances the peak current values in the differential pulse anodic stripping voltammetric (DPASV) determination of it at sub-nanomolar levels [25]. The developed method has also been satisfactorily applied for the determination of diltiazem in human serum and pharmaceutical samples.
In this study, we present an electroanalytical method based on anodic stripping voltammetry on a carbon paste electrode modified by Co3O4/SnO2 nanopowders for determination of trace amount of diltiazem in pharmaceutical and biological samples. The factors that affect on the performance of the DPASV were optimized and the proposed method was applied in the determination of diltiazem. In accordance with our resulting data, the Co3O4/SnO2 nanopowders offered several distinct advantages, including an extraordinary stability, reproducibility, low background response and a satisfactory detection limit for diltiazem.
Tin and cobalt-based oxides and complexes are universally known as inorganic or organometallic materials with wide electrocatalytic applications in modified electrodes, gas sensors, solar cells, and high-performance catalysts [26–29]. One of these usages is electro-catalytic role that applied in chemically modified electrodes. Some studies showed the electrocatalytic of Co or Sn-based materials such as cobalt phthalocyanine, [30] iron-doped cobalt oxide [31] or tin-modified palladium electrodes [32].
Experimental
Reagents and chemicals
All chemicals were of analytical grades and obtained from Merck (Germany). Diltiazem was purchased from Amin Pharmacy Corporation. Graphite powder (particle diameter 1–2 µm) and high-viscosity paraffin with high density was used as the pasting liquid for the carbon paste electrode purchased from Merck. Double distilled water was used throughout. Diltiazem stock solution (1.0 mM) was prepared by dissolving 0.0207 g of diltiazem separately in buffer phosphate solution in a 50 mL volumetric flask. Working solutions were prepared by appropriate dilution of the stock solution with buffer solution.
Apparatus
Electrochemical measurements were performed using a Metrohm 797 VA Potentiostat–Galvanostat. The experiments were carried out in a single-compartment three-electrode cell, at room temperature (25 °C). The counter electrode was a platinum wire, and an Ag/AgCl saturated KCl electrode was used as the reference electrode. Carbon paste electrodes modified with Co3O4/SnO2 nanopowders (MCPEs) were used as the working electrode. The preconcentration step was conducted using a magnetic stirrer at 200 rpm. The electrochemical experiments were performed in an electrolytic cell with 20 mL solutions. A digital pH meter (Metrohm 827) was used for the preparation of the buffer solutions in voltammetric experiments. A double water distiller with extremely pure distillation with conductivity of 1 µS/cm was used.
Procedures
Preparation of Co3O4/SnO2 nanopowders
The Co3O4/SnO2 nanopowders were prepared according to the similar procedure described previously [33]. For the synthesis of this compound, first 0.05 mol of cobalt acetate was mixed with 0.2 mol of melted stearic acid to produce cobalt stearate sol. In the next step 0.05 mol of tin tetrachloride was reacted to stoichiometric mol of n-butanol (in ice bath) to produce tin alkoxide solution. Then cobalt stearate sol was mixed with tin alkoxide solution for production of gel precursor. Afterwards the gel precursor was calcined to a temperature of 800 °C for 4 h. Finally during pulverization the Co3O4/SnO2 nanocatalyst was obtained.
Fabrication of Co3O4/SnO2 modified carbon paste electrode
Carbon paste electrodes (CPE) were prepared by mixing 68.9 % graphite powder with 8 % Co3O4/SnO2 nanopowders and 23.1 % paraffin oil (ratio of C:Paraffin: Co3O4/SnO2, 68.9:23.1:8, w/w) on mortar for at least 20 min to produce the modified carbon paste electrode (MCPE). The MCPE was finally obtained by packing the paste into an insulin syringe and arranged with a copper wire serving as an external electric contact. Appropriate packing was achieved by pressing the electrode surface against a weighing paper until a smooth surface was obtained.
General analytical procedure
Solutions (20 mL) containing appropriate amounts of diltiazem in 0.1 M phosphate buffer at pH 6.3 were transferred into the voltammetric cell. The differential pulse voltammograms were recorded by applying positive going potentials from 0.53 to 0.98 V. The voltammograms showed anodic peaks around 0.78 V corresponding to diltiazem of heights proportional to the concentrations in solution. The calibration curve was obtained by plotting anodic peak currents of diltiazem versus the corresponding concentrations. After each measurement, the MCPE was regenerated by pushing an excess of paste out of the tube, removing the excess, and mechanically polishing the electrode surface.
Real sample preparation for measurements
Twenty tablets were weighed accurately and finely powdered. A portion of the powder, equivalent to 0.232 g of diltiazem was transferred to a 100 mL volumetric flask and dissolved in approximately 90 mL of choloridric acid/water (1:10). The solution was then filtered and the first portion of the filtrate was discarded. An accurate measured volume of the filtrate was quantitatively diluted with buffer phosphate to yield a sample solution having a final concentration assumed to be 0.1 mM diltiazem. An aliquot was then transferred to a voltammetric cell containing 20 mL of buffer phosphate (pH 6.3) to yield a final concentration of 30 µM diltiazem.
For sample preparation of plasma, the plasma samples was diluted with 25 mM ammonium acetate (pH 5.0), then centrifuge 20 min at 8000 rpm to remove of proteins. Then 1 mL portion of plasma was added to 2 mL of acetonitril for remove excess of proteins. After mixing for 2 min and centrifugation for 10 min at 7000 rpm, the aqueous layer was discarded and the organic layer was evaporated to dryness under stream of nitrogen. The residues were reconstituted in phosphate buffer pH 6.3 so as the final concentration was in range (100–500 nM) and transferred to the voltammetric cell.
For preparation of urine sample, urine of a healthy person was filtered and was diluted with phosphate buffer (1:10). Other steps are similar to the plasma treatments.
Results and discussion
Characterization of Co3O4/SnO2 nanopowders
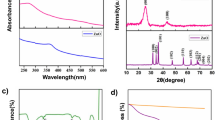
To analyse the crystal phases, XRD pattern was recorded. The obtained XRD pattern of Co3O4/SnO2 nanopowders after heat treatment in 800 °C in air for 4 h is shown in Fig. 2. The pattern includes two main phases, cobalt oxide (JCPDS: 78-1969) and tin oxide (JCPDS: 77-0452) phase. The particle size of crystallites (L) has been estimated with Scherrer formula [34] [Eq. (1)]:
where K is the form factor (equal to 0.9), λ = 0.15418 nM, 2θ is the peak position and Δ(2θ) is the full width at half maximum of the diffraction peak in terms of radians.
In this way, we obtained the crystallite size of the nanopowders calcined at 800 °C about 42 nM.
Electrochemical behavior at CPE and MCPE
Cyclic voltammograms of diltiazem were recorded at the phosphate buffer (pH 6.3) at both bare CPE and CPE modified with 8 % (w/w) Co3O4/SnO2 nanopowders. The voltammograms recorded in buffer solution at the bare CPE exhibited no any voltammetric peak over the entire pH range (Fig. 3, curve C) and in diltiazem solution (curve B). Whereas the voltammograms recorded in 100 nM diltiazem solution at the modified Co3O4/SnO2 nanopowders electrodes exhibited a single irreversible anodic peak over the entire pH range. However, this peak was sharp and better developed in phosphate buffer of pH 6.3 when using CPE modified with 8 % (w/w) Co3O4/SnO2 nanopowders (Fig. 3, curve A).
In addition, the electroactivity of the modified electrode is demonstrated in the differential pulse voltammograms (DPVs) observed for 100 nM diltiazem at the Co3O4/SnO2 modified electrode and the bare CPE in 0.1 M phosphate buffer with pH 6.3 (Fig. 4). At the modified electrode, the oxidation peaks became well defined and appeared at 780 mV. This enhancement in current responses is a clear evidence of the electroactivity effect of the modified electrode towards the oxidation of diltiazem.
Composition and stability of the MCPE
The effect of the carbon paste composition on the voltammetric response of the electrode modified with Co3O4/SnO2 nanopowders was evaluated by differential pulse voltammetry. Electrodes with different percent of modifier were prepared and examined for their voltammetric signals under identical conditions (Table 1). The maximum peak current was obtained for 8 % modified Co3O4/SnO2 nanopowders in the paste. Higher concentrations (>10 %) showed a decrease in the peak current. This is presumably due to the reduction of conductive area at the electrode surface. According to these results, a carbon paste composition of 8 % modified Co3O4/SnO2 nanopowders, 68.9 % graphite and 23.1 % paraffin oil was used in further studies.
Influence of accumulation time and accumulation potential
The influence of accumulation time is examined from 40 to 240 s. The DPV peak current of diltiazem increased with accumulation time increasing from 40 to 190 s, as shown in Fig. 5 for a 300 nM diltiazem solution. But when it exceeds 190 s, the peak current remains constant due to the surface saturation.
The influence of accumulation potential is examined from −0.1 to −0.5 V. However, with the potential increase to −700 mV more current to come, but since the peak current also increased in electrolyte, potential −200 mV as the optimal deposition potential due to the lower background and absence of any peaks in the electrolyte is selected.
The type of supporting electrolyte
The effect of different salt solutions (as supporting electrolyte) on DPV voltammogram of diltiazem was investigated. Sample solutions containing salts such as KCl, KNO3, NaNO3 and phosphate buffer in the concentration range of 0.01–0.2 mol L−1 were tested. The results revealed that the peak current and sensitivity of the method do not change significantly with type and salt concentration and the phosphate buffer was chosen as the best electrolyte.
The effect of pH on the peak current of diltiazem
The effects of the pH values of both the preconcentration and stripping solutions on the response of the Co3O4/SnO2 nanopowders to diltiazem were investigated. The response of the MCPE is strongly affected by these conditions (the pH or the ionic strength) in the ion exchange reactions. The effect of pH on the electrode response was tested in solutions of 300 nM diltiazem prepared at different pHs. Figure 6 shows the effect of the pH of the preconcentration solution on the anodic stripping peak current of diltiazem, and shows that the pH has a strong influence on the preconcentration process. The anodic stripping peak current of diltiazem increased with increasing pH over the range of 3.3–6.3, and reached a maximum level at pH 6.3. During this process, diltiazem selectively enters paste due to simple electrostatic attractive forces, since it exists as cation at pH 6.3. The anodic stripping peak current of diltiazem decreased with increasing pH at basic pH, which can be attributed to the deprotonation of diltiazem. The results indicated that the effect of the stripping solution pH on the response of Co3O4/SnO2 nanopowders-MCPE to diltiazem is similar behavior to that seen for the preconcentration solution.
Response profile for the Co3O4/SnO2 nanopowders-MCPE for preconcentration solutions at different pH values, containing 300 nM diltiazem; other conditions as Fig. 4
Linearity, limit of detection and limit of quantitation
Relationship between oxidation peak current magnitude and concentration of diltiazem was examined in phosphate buffer of pH 6.3 by the developed DP-ASV method utilizing the developed 8 % (w/w) Co3O4/SnO2 nanopowdes modified CPE. A linear dynamic range of 50–650 nM diltiazem was obtained following its preconcentration onto the developed 8 % (w/w) MCPE electrode by adsorption/accumulation for 190 s at −0.2 V (Fig. 7). Its corresponding regression equation was: i p (nA) = 1.136 C + 53.25, R 2 = 0.997. Limits of detection (LOD) and quantitation (LOQ) of bulk diltiazem were estimated 15 nM and 50.1 nM diltiazem based on 3 S/N and 10 S/N, respectively. The results indicated the reliability of the developed DP-ASV method for the trace assay of bulk diltiazem. This method shows a LOD of lower or compatible with other works such as high-performance liquid chromatography (0.9 µM), [13] and voltammetric sensor (0.5 nM) [35].
Differential pulse voltammetric responses for successive additions of diltiazem from 50 (a) to 650 nM (i) concentration (a) and the calibration curve (b) at the Co3O4/SnO2 (8 %) nanopowders modified CPE; other conditions: preconcentration time: 190 s; preconcentration potential: −200 mV; scan rate: 15 mV/s
Trueness and precision
Trueness and precision of the optimized DP-ASV method were evaluated by performing five replicate measurements for various concentrations of diltiazem (250, 350 and 550 nM) through intraday and inter-day assays following preconcentration by adsorptive accumulation onto MCPE at −0.2 V for 190 s. To characterize the precision of the modified electrode, repetitive determinations of diltiazem were carried out at 350 and 550 nM concentrations at 0.1 M phosphate buffer. The results of five replicate measurements showed a relative standard deviation of 3.2 and 2.5 % (relative standard deviation), respectively, indicating that the results are reproducible. Also for determination of trueness as percentage relative error (Er %) was performed five replicate measurement for 250 nM diltiazem that was 0.16 %.
Interferences
The influence of various foreign species and co-formulated drugs on the determination of 100 nM diltiazem was investigated. The tolerance limit was taken as the maximum concentration of the foreign substances, which caused an approximately ±5 % relative error in the determination. The results showed that no interference occurred for the co-formulated drugs such as atenolol, phenobarbital and phenytoin and common substances and ions such as Na+, Ca2+,K+, Ni2+, Fe3+, glucose, acid citric, urea, glycine, and ascorbic acid. The fact that Na+, K+, Ca2+, acid citric, glucose, urea, glycine, atenolol and phenobarbital (a 1000-fold excess) and ascorbic acid (a 100-fold excess) and phenytoin (a tenfold excess) were not interference is of particular significance.
Analytical application
Assay of tablet
The validity of the proposed voltammetric method was investigated by assaying diltiazem in tablets (each is labeled to contain 60 mg diltiazem per tablet). The recoveries were calculated by the calibration graph. The statistical calculations for the assay results show a good precision of the proposed method (Table 2).
Assay of diltiazem in spiked human urine
The proposed method was also successfully applied to the determination of diltiazem in spiked urine samples from healthy volunteers using calibration curve. This determination carried out at three different levels of concentrations (250, 300 and 420 nM) and six determinations were carried at each concentration level (Table 3). The mean apparent recoveries for the three concentrations were 101.4, 99.64 and 97.33 % with relative standard deviations of 4.9, 4.5 and 4.1 %, respectively.
Assay of diltiazem in spiked human plasma
The proposed method was applied to the determination of diltiazem in spiked human serum using the calibration curve. The direct determination of diltiazem in human serum was found to be possible after dilution of the sample with the supporting electrolyte. The percentage recovery of the drug in serum, based on the average of six replicate measurements, is listed in Table 4. The values obtained for recovery are acceptable for biological fluids.
Conclusion
The Anodic stripping voltammetry method (ASV) for the quantitative determination of diltiazem with carbon paste electrode modified with Co3O4/SnO2 nano-powder was found to be simple and highly sensitive over the reported data for the determination of diltiazem. It can be used successfully to stability-indicating assay the drug in dosage form as well as in urine and plasma at trace levels.
References
Feld, G., Singh, B.N.: Diltiazem-pharmacological properties and therapeutic uses. Hosp. Formul. 20, 814 (1985)
Hermann, Ph, Morselli, P.L.: Pharmacokinetics of diltiazem and other calcium entry blockers. Acta Pharmacol. Toxicol. 57, 10–20 (1985)
Kolle, E.U., Ochs, H.R., Vollmer, K.O.: Pharmacokinetic model of diltiazem. Arzneim.-Forsch. 33, 972–977 (1983)
Rovei, V., Gomeni, R., Mitchard, M., Larribaud, J., Blatrix, C., Thebault, J.J., Morselli, P.L.: Pharmacokinetics and metabolism of diltiazem in man. Acta Cardiol. 35, 35–45 (1980)
Bianchetti, G., Regazzi, M., Rondanelli, R., Ascalone, V., Morselli, P.L.: Bioavailability of diltiazem as a function of the administered dose. Biopharm. Drug Dispos. 12, 391–401 (1991)
Suleiman, M.S., Abdulhameed, M.E., Najib, N.M., Muti, H.Y.: Effect of ultraviolet radiation on the stability of diltiazem. Int. J. Pharm. 50, 71–73 (1989)
Laeer, S., Scholz, H., Uebeler, P., Neumann, J., Zimmermann, N.J.: Quantitation of diltiazem in human cardiac tissue using high-performance liquid chromatography. Chromatogr. Sci. 35, 93–96 (1997)
Meehan, E., Kelly, J.G.: High-performance liquid chromatographic assay for diltiazem in small-volume blood specimens and application to pharmacokinetic studies in rats. J. Chromatogr. 729, 297–300 (1996)
Morris, R.G., Saccoia, N.C., Jones, T.E.: Modified liquid chromatographic assay for diltiazem and metabolites in human plasma. Liq. Chromatogr. J. Relat. Technol. 19, 2385–2394 (1996)
Shi, X.J., Zong, M.K., Wang, H.T., Zhang, J.H.: Studies on the chemical components of essential oils of Elsholtzia patrini Garcke. Yaowu Fenxi Zazhi 15(23), 20–22 (1995)
Ascalone, V., Locatelli, M., Malavasi, B.J.: Determination of diltiazem and its main metabolites in human plasma by automated solid-phase extraction and high performance liquid chromatography: a new method overcoming instability of the compounds and interference problems. Chromatogr. B Biomed. Appl. 657, 133–140 (1994)
Li, N., Li, C.-L., Lu, N.-W., Dong, Y.-M.: A novel micellar per aqueous liquid chromatographic method for simultaneous determination of diltiazem hydrochloride, metoprolol tartrate and isosorbide mononitrate in human serum. J. Chromatogr. B Biomed. Appl. 967, 90–97 (2014)
Christensen, H., Carlson, E., Asberg, A., Schram, L., Berg, K.J.: A simple and sensitive high-performance liquid chromatography assay of diltiazem and main metabolites in renal transplanted patients. Clin. Chim. Acta 283, 63–75 (1999)
Devarajan, P.V., Dhavse, V.V.: High-performance thin-layer chromatographic determination of diltiazem hydrochloride as bulk drug and in pharmaceutical preparations. J. Chromatogr. B 706, 362–366 (1998)
Alebic-Kolbah, T., Plavsic, F.: Determination of serum diltiazem concentrations in a pharmacokinetic study using gas chromatography with electron capture detection. J. Pharm. Biomed. Anal. 8, 915–918 (1990)
Sreedhar, K., Sastry, C.S.P., Narayane Reddy, M., Sankar, D.G.: Extractive spectrophotometric determinations of diltiazem hydrochloride. Indian Drugs 32, 90–92 (1995)
Rahman, N., Azmi, S.N.H.: Spectrophotometric determination of diltiazem hydrochloride with sodium metavanadate. Microchem. J. 65, 39–43 (2000)
Hubert, Ph, Chiap, P.J.: Automatic determination of diltiazem and desacetyldiltiazem in human plasma using liquid–solid extraction on disposable cartridges coupled to HPLC—part I: optimization of the HPLC system and method validation. J. Pharm. Biomed. Anal. 9, 877–882 (1991)
Ghandour, M.A., Aboul Kasim, E., Ali, A.M.M., El-Haty, M.T., Ahmed, M.M.: Adsorptive stripping voltammetric determination of antihypertensive agent: diltiazem. J. Pharm. Biomed. Anal. 25, 443–451 (2001)
Javanbakht, M., Divsar, F., Badiei, A., Fatollahi, F., Khaniani, Y., Ganjali, M.R., Norouzi, P., Chaloosi, M., Mohammadi Ziarani, G.: Determination of picomolar silver concentrations by differential pulse anodic stripping voltammetry at a carbon paste electrode modified with phenylthiourea-functionalized high ordered nanoporous silica gel. Electrochim. Acta 54, 5381–5386 (2009)
Javanbakht, M., Khoshsafar, H., Ganjali, M.R., Norouzi, P., Adib, M.: Adsorptive stripping voltammetric determination of nanomolar concentration of cerium(III) at a carbon paste electrode modified by N′-[(2-Hydroxyphenyl)Methylidene]-2-Furohydrazide. Electroanalysis 21, 1605–1610 (2009)
Javanbakht, M., Khoshsafar, H., Ganjali, M.R., Norouzi, P., Badei, A., Hasheminasab, A.: Stripping voltammetry of cerium(IIl) with a chemically modified carbon paste electrode containing functionalized nanoporous silica gel. Electroanalysis 20, 203–206 (2008)
Javanbakht, M., Khosh safar, H., Ganjali, M.R., Badei, A., Norouzi, P., Hasheminasab, A.: Determination of nanomolar mercury(II) concentration by anodic-stripping voltammetry at a carbon paste electrode modified with functionalized nanoporous silica gel. Cur. Anal. Chem. 5, 35–41 (2009)
Javanbakht, M., Fathollahi, F., Divsar, F., Ganjali, M.R., Norouzi, P.: A selective and sensitive voltammetric sensor based on molecularly imprinted polymer for the determination of dipyridamole in pharmaceuticals and biological fluids. Sens Actuators B: Chem 182, 362–367 (2013)
Attaran, A.M., Javanbakht, M., Fathollahi, F., Enhesari, M.: Determination of salbutamol in pharmaceutical and serum samples by adsorptive stripping voltammetry on a carbon paste electrode modified by iron titanate nanopowders. Electroanalysis 24, 2013–2020 (2012)
Taylor, D., Fleig, P.F., Page, R.A.: Characterization of nickel titanate synthesized by sol–gel processing. Thin Solid Films 408, 104–110 (2002)
Martinez, A.A.: Cerium–terbium mixed oxides as potential materials for anodes in solid oxide fuel cells. J. Power Sources 151, 43–51 (2005)
Chaubal, N.S., Sawant, M.R.: Synergistic role of aluminium in stabilization of mixed metal oxide catalyst for the nitration of aromatic compounds. Catal. Commun. 7, 443–449 (2006)
Xiong, W., Kale, G.M.: Novel high-selectivity NO2 sensor incorporating mixed-oxide electrode. Sens. Actuators, B 114, 101–108 (2006)
Li, B., Wang, M., Zhou, X., Wang, X., Liu, B., Li, B.: Pyrolyzed binuclear-cobalt-phthalocyanine as electrocatalyst for oxygen reduction reaction in microbial fuel cells. Bioresour. Technol. 193, 545–548 (2015)
Zhang, J., Wang, X., Qin, D., Xue, Z., Lu, X.: Fabrication of iron-doped cobalt oxide nanocomposite films by electrodeposition and application as electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 320, 73–82 (2014)
Antonin, V.S., Assumpção, M.H.M.T., Silva, C.M., Parreira, L.S., Lanza, M.R.V., Santos, M.C.: Synthesis and characterization of nanostructured electrocatalysts based on nickel and tin for hydrogen peroxide electrogeneration. Electrochim. Acta 109, 245–251 (2013)
Enhessari, M., Parviz, A., Ozaee, K., Karamali, E.: Magnetic properties and heat capacity of CoTiO3 nanopowders prepared by stearic acid gel method. J. Exp. Nanosci. 5, 61–68 (2010)
Enhessari, M., Parviz, A., Karamali, E., Ozaee, K.: Synthesis, characterisation and optical properties of MnTiO3 nanopowders. J. Exp. Nanosci. 7, 327–335 (2012)
Gevaerd, A., Caetano, F., Oliveira, P., Zarbin, A., Bergamini, M., Marcolino-Junior, L.: Thiol-capped gold nanoparticles: influence of capping amount on electrochemical behavior and potential application as voltammetric sensor for diltiazem. Sens. Actuators B: Chem. 220, 673–678 (2015)
Acknowledgments
We gratefully acknowledge the Payame Noor University (Delijan, Iran) and the Amirkabir University of Technology (Tehran, Iran) for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Attaran, A.M., Abdol-Manafi, S., Javanbakht, M. et al. Voltammetric sensor based on Co3O4/SnO2 nanopowders for determination of diltiazem in tablets and biological fluids. J Nanostruct Chem 6, 121–128 (2016). https://doi.org/10.1007/s40097-015-0186-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-015-0186-6