Abstract
Solanum nigrum, a medicinal plant, traditionally used in treating diabetes mellitus. In this study, we used the leaf extract of the plant to synthesize silver nanoparticles (AgNPs), as a proposition to treat alloxan-induced diabetic rats. The phytosynthesised AgNPs were analyzed using UV–visible and Fourier transform infra-red spectroscopy for their functional groups. Transmission electron microscopy revealed that, the synthesized particles are found to be 4–25 nm in size. Monodispersed and spherical nature of synthesized AgNPs were shown by scanning electron microscope and the presence of Ag in the AgNPs was confirmed by energy dispersive spectrum. The phytosynthesised AgNPs were evaluated for its antidiabetic activity in alloxan-induced diabetic rats. AgNPs-treated diabetic rats found to be significantly improved the dyslipidemic condition as seen in the diabetic control. Furthermore, it also reduced the blood glucose level over the period of treatment. The improvement in body weight was also found to be evidence for S. nigrum extract-mediated AgNPs as a potential antidiabetic agent against alloxan-induced diabetic rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a group of syndromes characterized by hyperglycemia; altered metabolism of lipids, carbohydrates, and proteins; and an increased risk of complications from vascular disease that affects 10 % of the population [1, 2]. Despite, the availability and extensive utilization of hypoglycemic agents, diabetes and the related complications continue to be major health concerned worldwide [3]. According to International Diabetic Federation the estimated diabetes prevalence in 2010 has risen to 285 million, representing 6.4 % of the world’s adult population, with a prediction that by 2030, the number of people with diabetes will have risen to 438 million, with this alarming concern, India has been declared as the “Diabetic capital of world”. Currently 40.9 million people in India suffering from diabetes and by 2030 there would be 79.44 million diabetics in India alone. It is also estimated that by the year 2030, diabetes is likely to be the seventh leading cause of death, accounting 3.3 % of total deaths in the world [4].
Owing to the progressive nature of the disease, an improved treatment strategy is required, which includes, the discovery of new drugs [5]. In addition, drug treatment, is not completely successful with diabetes and there is a compelling need for better prevention and treatment strategies [6]. Nevertheless, traditionally people have been using many plants to treat diabetes empirically despite the lack of safety and efficacy [7]. At this juncture, it is necessary to test the herbal medicine as an alternative to synthetic agent [8].
In the search of new opportunities for treatment of DM, the current study has focused on the efficacy of S. nigrum. The plants belong to the Solanaceae family, are considered as poisonous to human, however, S. nigrum is an edible plant and possesses antioxidant and hepatoprotective activity [9]. Furthermore, many parts of this plant are used as a traditional medicine. Despite, it has been used as an antidiabetic agent in traditional medicine, it lacks scientific evidence [10]. In addition, this is the first of its kind reporting the efficiency of S. nigrum-mediated AgNPS as an anti-hyperglycemic agent.
Since, the last decade the applications of AgNPs have been increasing rapidly in various fields. Originally, the silver metal was used as an anti-microbial agent, later in the 1990’s it has been used in the medicinal field as silver colloids to treat various diseases [11]. AgNPs are usually a cluster of particles ranging between 1 and 100 nm in size and they exhibit new properties based on their size, distribution and morphology [12]. Nanoparticles are becoming the focus of intensive research, due to their wide range of applications in areas such as catalysis, optics, antimicrobials, and biomaterial production [13, 14].
Various approaches have been used for the synthesis of AgNPs. The phytosynthesis approach has many advantages over chemical, physical, and microbial synthesis [15–18], as there is no need of the elaborated process of culturing and maintaining the cell, hazardous chemicals and high-energy requirements.
In the present study, we have characterized the S. nigrum leaf extract-mediated AgNPs and assessed it, as an antidiabetic agent in alloxan-induced diabetic rat model.
Materials and methods
Collection of plants
The S. nigrum plant material was collected from Erode District, Tamil Nadu, India and authenticated by Dr. S. ArunPrakash, Department of Botany, Government Arts College, Namakkal, Tamil Nadu, India.
Preparation of aqueous extract
The leaves of the plants collected were washed and air dried in shade at room temperature for 7 days. The air dried plants were pulverized in an electric grinder and sieved using mesh to obtain uniform size for further study. The extraction process was carried out with the help of Soxhlet apparatus. Fifteen grams of dry powder were subjected to soxhlet extraction with 300 mL methanol, the extraction was repeated up to 10 cycles at 45 °C to ensure the complete recovery.
Synthesis of AgNPs
AgNPs synthesis was carried out according to Aravinthan et al. [19]. Briefly, 4 mL of the aqueous extract was mixed with 96 mL of 1 mM AgNO3 solution and the resulting greenish white mixture was incubated for 8 h in a rotary shaker (200 rpm) at 26 °C. Reduction of Ag+ ions to Ag nanocrystals was monitored by a change in color of the reaction mixture from greenish white to dark brown [19].
Characterization of AgNPs
Surface Plasmon resonance (SPR) bands of the synthesized AgNPs were characterized using UV–Vis spectroscopy (Shimadzu UV-2450) in the range of 200–600 nm. Morphological analysis and the crystalline nature of the particles were investigated by transmission electron microscopy (TEM) using FEI Tecnai TF 20 high-resolution TEM instrument operated at an accelerating voltage of 200 kV [20]. The characterization of the synthesized AgNPs was conducted with X-ray diffractometer (XPERT-Pro diffractometer using Cu Kα radiation), operated at 2θ from 30 to 80° at 0.041°/min with a time constant of 2 s. AgNPs synthesis and the elemental composition were further confirmed by SEM–EDS (SEM–EDS; JEOL-64000, Japan). The chemical characterization of changes in the surface and surface composition was performed by Fourier transform infrared spectroscopy (Shimadzu) within the mid IR region of frequency 4000–400 cm−1 [19].
Induction of diabetes
Diabetes was induced in male Wistar albino rats aged more than 8 weeks (140–160 g body weight) by single intraperitoneal administration of alloxan (single dose of 200 mg/kg body weight). Within 48 h after alloxan administration, blood glucose concentrations were measured via tail clip sampling. Animals with a blood glucose concentration 200 mg/dL were considered to be diabetic [21].
Toxicity assessment in rat
Acute toxicity test on AgNPs was performed in experimental rats with a graded dose levels of AgNPs (10 and 20 mg/kg body weight) [22]. The rats were observed continuously for 2 h for behavioral, neurological and autonomic profiles and after a period of 24 and 72 h for any lethality or death. Further experiments were carried out using 10 mg/kg body weight AgNPs.
Experimental design
The animals were divided into five groups and each group consisted of 5 rats. Group I: Untreated normal rats; (Normal control, received only distilled water) Group II: Diabetic control, alloxan 200 mg/kg single dose, received no treatment; Group III: Diabetic rats, received glibenclamide 0.5 mg/kg for 21 days; Group IV: Diabetic rats, received S. nigrum Methanol extract 10 mg/kg for 21 days; Group V: Diabetic rats, received phytosynthesised AgNPs 10 mg/kg for 21 days.
Oral glucose tolerance test
The oral glucose tolerance test was performed after 21 days administration of AgNPs and plant extract to the respective groups. The rats were fasted overnight, and 2 g/kg glucose was administered orally and the blood was collected from the tail vein prior and the post administration of glucose at 0,30,60,90, and 120 min and fasting blood glucose level was measured using a glucometer (Accu-Chek, Roche Diagnostic, Manheim, Germany).
Biochemical analysis
Blood samples were collected by retro orbital puncture and centrifuged at 1000×g for 15 min and the collected serum was stored at −80 °C until analysis. Total cholesterol and Triglycerides were estimated using (Biovision, Milpitas, CA, USA) kit as manufacture’s instruction.
Statistical analysis
All the data were expressed as mean ± SD, and the statistical significance between the groups was analyzed using one-way ANOVA followed by Tukey’s post hoc test. If P ≤ 0.05 were considered significant.
Results and discussion
Synthesis and characterization of AgNPs
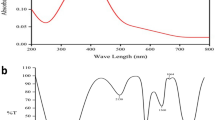
The S. nigrum leaf extract was mixed in the aqueous solution of the silver ion complex, and became yellowish brown due to the reduction of silver ion, indicating the completion of the reaction. Similar changes in color have also been reported earlier [23, 24]. The AgNps exhibit yellowish brown color in aqueous solution owing to excitation of surface plasmon vibrations in AgNPs [25, 26]. The plant leaf extract known to posses many active compounds such as, sugar [27] caffeine and theophylline [28] and antioxidants [29] are reported to involve in the formation of AgNPs. As S. nigrum also contains high antioxidants, we speculate this AgNPs formation due to the presence of high amount of antioxidants in the extract [9]. The Fig. 1a represents the absorbance spectrum of AgNPs formed at various times (0, 2, 4, 6 h) and the maximum absorption peak was observed in the range of 370–420 nm. It is reported that the absorption spectrum of spherical AgNPs present at 420 nm, and maximum absorption peak shift towards the blue region directly correlates with smaller particle size, and providing the clue for the size of the synthesized nanoparticle in the range of ≤25 nm [30]. Figure 1b shows a representative TEM image of phytosynthesized AgNPs. The AgNPs were seen to be spherical and monodispersed with the size range of 4–25 nm. This result is well agreed with the UV–Vis spectra result, in which the maximum peak shifts towards the blue region (λ max at 420 nm), and providing the direct evidence for size [30].
The crystalline nature of the silver nanoparticles of S. nigrum, the XRD analysis was undertaken, Fig. 2a revealing six peaks at degree (2θ) 10.00, 11.51, 12.00, 27.75, 32.15, and 45.94 corresponding to six diffraction facets of silver. The broadening of X-ray peaks observed can be attributed to the organic content of leaf extract. Analysis of AgNPs through Energy dispersive X-ray (EDX) spectrometers confirmed the presence of elemental silver signal at 3 keV along (Fig. 2b) with other weak signals corresponding to oxygen and carbon, which could have essentially derived from the plant extract and consistent with previous studies [31, 32]. FTIR measurement was carried out to identify the possible biomolecules in S. nigrum leaf extract responsible for capping leading to efficient stabilization of the silver nanoparticles (Fig. 3). The IR spectrum of silver nanoparticles manifests prominent absorption bands located at 3282, 2926, 2357, 1670, 1523, 1240, 1064, 667, and 422.41 cm−1. The bands seen in 3282 and 2926 cm−1 represent the stretching vibrations of primary and secondary amines, respectively. The peak at 2357 could be attributed to H bonded OH stretching of a secondary metabolites present in the extract which might act as a reducing agent to synthesize AgNPs [33].
The antidiabetic activity of phytosynthesized AgNPs
An acute toxicity study revealed the non-toxic nature of the methanolic extract and green synthesized AgNPs. The rats treated with different doses of S. nigrum did not show any drug-induced physical signs of toxicity during the whole experimental period, and no deaths were observed. Based on the acute toxicity studies, with a varying concentration (10, 20 mg/kg) of AgNPs, 10 mg/kg body weight was fixed as an optimal concentration, and thus used in the treatment of alloxan-induced diabetic rats.
The blood glucose level of each group has been estimated during the course of treatment (Fig. 4a), to evaluate anti-hyperglycemic effect of plant extract and phytosynthesized AgNPs. At 14 and 21 days the treated groups (extract/AgNPs) pronouncedly decrease the blood glucose level compared to that of diabetic control (Group II). AgNPs were shown to have reduced blood glucose level higher than plant extract alone. On the other hand glibenclamide-treated groups significantly reduced the blood glucose level throughout the period of treatment compared to group II, IV and V.
a Effect of oral administration of S. nigrum leaf extract and AgNPs at a dose of 10 mg/kg, in Blood glucose level of alloxan-induced diabetic rats before and after treatment. Each column represents mean ± SD for five rats. *P < 0.05; **P < 0.01; ***P < 0.001 different from diabetic control. b Effect of S. nigrum leaf extract and AgNPs in oral glucose tolerance test after 21 days of treatment. Each column represents mean ± SD (n = 5). All the values were found to be statistically significant compared to diabetic control at *P < 0.05
The glucose tolerance level of S. nigrum leaf extract-mediated AgNPs-treated group was evaluated in diabetic rats (Fig. 4b), by estimating its efficacy in reducing hyperglycemic condition in blood followed an administration of glucose orally, and compared it with standard drug (Glibenclamide 0.5 mg/kg). The phytosynthesized AgNPs-treated animals shown to have a significant improvement in lowering blood glucose compared to Group II. Among the treated groups the efficiency of compounds in lowering the blood glucose level over a period of 2 h has followed with an order, group III > group V > group IV. Phytosynthesized AgNPs treatment is shown to improve the blood glucose level which is comparable to glibenclamide-treated groups. Alloxan-induced diabetic rat model has been widely used in several studies [34, 35]. In addition, the mode of action of alloxan is well documented [36]. In the treatment of diabetes, blood glucose concentration is considered as a routine and major biochemical marker to monitor the improvement in the disease condition, the results obtained for blood glucose concentration of treated groups showed a smaller total area under the curve which is significantly lesser than the diabetic control group, and these findings are in accordance with previous research [36]. Figure 5 demonstrates the level of changes in body weight of experimental rats at before and after treatment. Weight loss is one of the major syndrome associated with diabetes, probably due to muscle wasting [37]. In our study the diabetic-induced rat group (Group II) showed significant weight loss (Fig. 5) Compared to group I (Normal control). The total cholesterol (TC) and triglycerides (TG) levels were also elevated in diabetic rats (Fig. 6a, b). It is well documented that DM tends to reduce body weight as a result of increased muscle wasting, dehydration, and fat catabolism. Whereas, the treatment with plant extract as well as phytosynthesized AgNPs significantly improved the body weight loss. The TC and TG levels were retrieved comparable to normal group over a period of 21 days administration of AgNPs, the mechanism of preventing the muscle loss could probably attribute to reversal of antagonism [38].
Conclusion
In this present study, AgNPs were rapidly synthesized using aqueous leaf extract of S. nigrum as a bio-reductant. The efficacy of phytosynthesized AgNPs as an anti-hyperlipidemic agent has been evaluated. This study revealed that the AgNPs are found to be safe and environmentally friendly, hence, these AgNPs can be considered in treating diabetes associated syndrome.
References
Alvin, C.P., David Allesio, D.D.: Endocrine pancreas and pharmacotherapy of diabetes mellitus and hypoglycaemia. In: Brunton, L., Chabner, B., Knollman, B. (eds.) Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 12th edn, p. 1237. McGraw-Hill, New York (2011)
Foster, D.W.: Diabetes Mellitus Harrison’s Principles of Internal Medicine, pp. 1979–1981. McGraw Hill, USA (1994)
El-Amrani, F., Rhallab, A., Alaoui, T., El-Badaoui, K., Chaki, S.: Hypoglycaemic effect of Thymelaea hirsuta in normal and streptozotocin-induced diabetic rats. J. Med. Plants Res. 3(9), 625–629 (2009)
Singh, U., Kochhar, A., Singh, S.: Blood glucose lowering potential of some herbal plants. J. Med. Plants Res. 5(19), 4691–4695 (2011)
Modi, P.: Diabetes beyond insulin: review of new drugs for treatment of diabetes mellitus. Curr. Drug Discov. Technol. 4, 39–47 (2007)
Baynes, J.W., Thorpe, S.R.: The role of oxidative stress in diabetic complications. Endocrinol. J. 3, 277–284 (1996)
Alarcon-Aguilara, F.J., Roman-Ramos, R., Perez-Gutierrez, S., Aguilar-Contreras, A., Contreras-Webar, C.C., Felores-Saenz, J.L.: Study of the anti-hyperglycemic effect of plants used as antidiabetics. J. Ethnopharmacol. 61, 101–110 (1998)
Grover, J.K., Yadav, S., Vats, V.: Medicinal plants of India with antidiabetic potential. J. Ethanopharmacol. 81, 81–100 (2002)
Jain, R., Sharma, A., Gupta, S., Sarethy, I.P., Gabrani, R.: Solanum nigrum: current perspectives on therapeutic properties. Altern. Med. Rev. 6, 78–85 (2011)
Sohrabipour, S., Kharazmi, F., Soltani, N., Kamalinejad, M.: Effect of the administration of Solanum nigrum fruit on blood glucose, lipid profiles, and sensitivity of the vascular mesenteric bed to phenylephrine in streptozotocin-induced diabetic rats. Med. Sci. Monit. Basic Res. 19, 133–140 (2013)
Kenneth, K.Y., Liu, X.: Silver nanoparticles—the real “silver bulltet” in clinical medicine? Med. Chem. Comm. 1, 125–131 (2010)
Satyavani, K., Gurudeeban, S., Ramanathan, T., Balasubramanian, T.: Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.). Schrad. J. Nanobiotechnol. 9, 43 (2011)
Kalimuthu, K., Babu, R.S., Venkataraman, D., Bilal, M., Gurunathan, S.: Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloid Surf. B. 65, 150–153 (2008)
Smitha, S.L., Nissamudeen, K.M., Philip, D., Gopchandran, K.G.: Studies on surface plasmon resonance and photoluminescence of silver nanoparticles. Spectrochim. Acta Mol. Biomol. Spectros. 71, 186–190 (2008)
Liu, Y.C., Lin, L.H.: New pathway for the synthesis of ultrafine silver nanoparticles from bulk silver substrates in aqueous solution by sonoelectrochemical methods. Electrochem. Commun. 6, 78–86 (2004)
Bae, C.H., Nam, S.H., Park, S.M.: Formation of silver nanoparticles by laser ablation of a silver target in NaCl solution. Appl. Surf. Sci. 197, 628–634 (2002)
Basavaraja, S., Balaji, D.F., Lagashetty, A., Rajasab, A.H., Venkataraman, A.: Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. Bull. 43, 1164–1170 (2008)
Jha, A.K., Prasad, K.: Green synthesis of silver nanoparticles using Cycas leaf. Int. J. Green Nanotechnol.: Phys. Chem. 1, 110–117 (2010)
Aravinthan, A., Govarthanan, M., Selvam, K., et al.: Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and rat splenocyte cytotoxic effects. Int. J. Nanomed. 10, 1977–1983 (2015)
Mukunthan, K.S., Elumalai, E.K., Patel, T.N., Murty, V.R.: Catharanthus roseus: a natural source for the synthesis of silver nanoparticles. Asian Pac. J. Trop. Biomed. 1(4), 270–274 (2011)
Tanquilut, N.C., Tanquilut, M.A.C., Torres, E.B., Rosario, J.C., Reyes, B.A.S.: Hypoglycemic effect of Lagerstroemia speciosa (L.) Pers. on alloxan-induced diabetic mice. J. Med. Plants Res. 3, 1066–1071 (2009)
Ghosh, M.N.: Fundamentals of Experimental Pharmacology, 2nd edn, pp. 153–158. Scientific Book Agency, Culcutta (1984)
Singhal, G., Bhavesh, R., Kasariya, K., Sharma, A.R., Singh, R.P.: Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanoparticle Res. 13, 2981–2988 (2011)
Banerjee, P., Satapathy, M., Mukhopahaya, A., Das, P.: Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 1, 3 (2014)
Maiti, S., Barman, G., Konar, L.J.: Synthesis of silver nanoparticles having different morphologies and its application in estimation of chlorpyrifos. Adv. Sci. Focus. 1, 145–150 (2013)
Barman, G., Samanta, A., Maiti, S., Konar, L.J.: Detection of Cu+2 ion by the synthesis of bio-mass-silver nanoparticle nanocomposite. Int. J. Sci. Eng. Res. 6, 1086–1097 (2014)
Shankar, S.S., Rai, A., Ahmad, A., Sastry, M.: Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 275, 496–502 (2004)
Krishnaraj, C., Jagan, E.G., Rajasekar, S., Selvakumar, P., Kalaichelvan, P.T., Mohan, N.: Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B 76, 50–56 (2010)
Ramteke, C., Chakrabarti, T., Saranki, B.K., Pandey, R.A.: Synthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. J. Chem. (2013). doi:10.1155/2013/278925
Martinez-Castanon, G.A., Nino-Martinez, N., Martinez-Gutierrez, F., Martinez-Mendoza, J.R., Ruiz, F.: Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 10, 1343–1348 (2008)
Govarthanan, M., Selvankumar, T., Manoharan, K., et al.: Biosynthesis and characterization of silver nanoparticles using panchakavya, an Indian traditional farming formulating agent. Int. J. Nanomed. 9, 1593–1599 (2014)
Lee, K.J., Park, S.H., Govarthanan, M., et al.: Synthesis of silver nanoparticles using cow milk and their antifungal activity against phytopathogens. Mater. Lett. 105, 128–131 (2013)
Dubey, S.P., Lahtinen, M., Särkkä, H., Sillanpää, M.: Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloids. Colloids Surf. B 80, 26–33 (2010)
Attanayake, A.P., Jayatilaka, K.A.P.W., Pathirana, C., Mudduwa, L.K.B.: Study of antihyperglycaemic activity of medicinal plant extracts in alloxan induced diabetic rats. Anc. Sci. Life 32, 193–198 (2013)
Ragini, V., Prasad, K.V., Bharathi, K.: Antidiabetic activity of Shorea tumbuggaia rox. Int. J. Innov. Pharm. Res. 2, 113–121 (2011)
Fröde, T.S., Medeiros, Y.S.: Animal models to test drugs with potential antidiabetic activity. J. Ethnopharmacol. 115, 173–183 (2008)
Swanston-Flatt, S.K., Day, C., Bailey, C.J., Flatt, P.R.: Traditional plant treatment for diabetes: studies in normal and streptozotocin diabetic mice. Diabetologia 33, 462–464 (1990)
Whitton, P.D., Hems, D.A.: Glycogen synthesis in perfused liver of streptozotocin diabetic rats. Biochem. J. 21, 150–153 (1975)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sengottaiyan, A., Aravinthan, A., Sudhakar, C. et al. Synthesis and characterization of Solanum nigrum-mediated silver nanoparticles and its protective effect on alloxan-induced diabetic rats. J Nanostruct Chem 6, 41–48 (2016). https://doi.org/10.1007/s40097-015-0178-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-015-0178-6