Abstract
Degradation rates and removal efficiencies of different parabens, namely, methylparaben, ethylparaben, propylparaben, and butylparaben using HO/Fe advanced oxidation process are studied in this work. With the aim of optimizing the removal of parabens from waters through the Fenton process, a factorial central composite orthogonal and rotatable design (FCCORD) was used. HO and Fe ion initial concentrations were selected as independent variables. The experimental procedure planned according to the FCCORD makes it possible to optimize the removal. The occurrence of interactions between these two variables can also be analyzed with the aid of the experimental design. Fenton process provides conversion efficiencies comprising between 85 and 94 % after a reaction time of 48 h, which reveals the appropriateness of this procedure for the removal of parabens from aqueous matrices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pharmaceuticals and personal care products (PPCPs) constitute a novel group of water pollutants. The presence of these chemicals in ground- and surface water has been repeatedly reported [1–3]. Many "emerging pollutants" can be regarded as recalcitrant compounds, since they cannot be eliminated in wastewater treatment plants through routine biological, chemical or physical treatment. Parabens is the common name of a wide variety of alkyl esters of p-hydroxybenzoic acid. Many of them find application as ingredients of pharmaceuticals, personal hygiene/care products and cosmetics in general [4]. Studies on the causes of breast cancer confirm that these kinds of products can be assimilated by the human body and reach tissues [5, 6]. Hence, the discernment of these pollutants in humans that have been in contact with them in their environments has attracted the interest of the scientific community. For instance, parabens have been found in fluids such as urine [7, 8], milk [9], serum [10] and semen [11, 12].
Since these chemicals may cause severe damages to health, the development of highly effective removal methods is receiving a great deal of attention in the last years. The use of processes such as adsoprtion [13, 14] or biosorption [15] have demonstrated to be effective in the treatment of waters polluted with parabens. The main advantage of this kind of processes is their relatively low cost. However, in adsorption or biosorption the pollutants are extracted from the liquid phase and concentrated on the surface of the solid adsorbent. This causes severe problems related with the wastes generated in the process. The so-called advanced oxidation processes (AOPs) appear as an attractive alternative to solve this problem, since in AOPs the pollutants are degradated to form smaller molecules (ideally CO and water). AOPs such as ozonation [16, 17] or UV-oxidation [18] have also been succesfully applied for the removal of parebens. These processes are highly efficient, their high cost being theri most important disadvantage. On the contrary, Fenton process is a promising technology [19, 20]. The main advantages of this procedure are technological simplicity, excellent results in pollutant removal, inexpensiveness and safety [21, 22]. The chemical basis of the Fenton process lays on the decomposition of HO at low pHs in the presence of Fe ions. The global process consists of several steps:
The main goal of this work is to analyze the feasibility of using the Fenton oxidation process to achieve the simultaneous degradation of four parabens present in aqueous matrices. With this purpose, a statistical design of experiments has been used to analyze the influence of two operational parameters (namely, HO and Feinitial concentrations) on the removal efficiency of four parabens. Optimal treatment conditions have been determined as well.
Methods
Chemicals
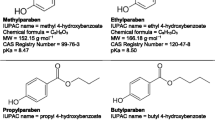
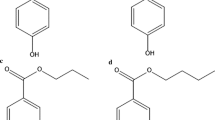
Methylparaben (MP, CHO), ethylparaben (EP, CHO), propylparaben (PP, CHO) and butylparaben (BP, CHO) were purchased from Sigma-Aldrich, with purities above 98 % in all cases. The molecular structures of parabens are illustrated in Fig. 1. Solutions containing an admixture of the four parabens (5 ppm each) were prepared using high-purity Millipore Milli-Q water. The pH of the solutions was kept constant by using a HClO/ClO buffer. HO (33 % w/v) and FeSO 7HO (analytical grade) were purchased from Merck (White House Station, NJ, USA).
Experimental procedure
All experiments were performed in a 250 mL glass reactor that was placed inside a thermostatic (25 0.5C) bath provided with a magnetic stirring system. 150 mL of aqueous solution of parabens was used in all experiments. As indicated above, an HClO/ClO buffer was used to keep pH constant. The adequate quantity of Fe(II) sulfate was incorporated. Finally, the required amount of hydrogen peroxide was added to make the reaction start.
With the aim of determining the period of time required to reach equilibration, several kinetic experiments were performed. In all cases the reaction was quenched at different time intervals previously set by adding NaHSO. Equilibration times comprised between 24 and 48 h were determined for the four pollutants. Hence, a reaction time of 48 h was used when performing the different experiments.
Analytical methods
Solutions consisting of an admixture of the four parabens were used. The concentrations of methylparaben, ethylparaben, propylparaben and buthylparaben present in the samples were analyzed simultaneously with the aid of a Waters HPLC chromatograph. A photodiode Array 996 detector and a Waters Nova-Pak C-18 column (5 m, 150 mm × 3.9 mm) were used. Well-defined peaks were obtained for the four chemicals under study, with retention times of 2.2, 2.8, 4.2 and 6.8 min for MP, EP, PP and BP, respectively. For each of the analyses, 100 L of solution was introduced (1 mL/min) into the chromatograph. The mobile phase was constituted by a mixture of methanol:water 60:40, containing 210 mol L phosphoric acid). Isocratic operation mode was chosen, that is, the proportion of the mobile phase was kept constant throughout the experiment.
Design of experiments
The use of an experimental design is an excellent choice to analyze if two or more independent variables—and their possible interactions—exert a statistically significant effect on the response variable. Furthermore, it makes it possible to minimize the number of experiments necessary with such an aim. Optimization of the process is also possible. The application of RSM provides a mathematical relationship between variables and experimental data can be fitted to a polynomial equation as follows [23].
where Y is the predicted response, is the offset term, is the linear effect, is the first-order interaction effect and is the squared effect. For the present study, a composite, central, orthogonal and rotatable design of experiment (CCORD) has been used. This kind of experimental design has been widely used in similar works. The number of runs,N, is:
wherek represents the number of independent variables (or factors) andn is the number of replicates of the central experiment.
To perform this study, a CCORD was used with two independent variables (namely, the initial concentrations of HO and Fe). The central experiment was replicated eight times,N being thus equal to 16.
To be used in the statistical calculations, the natural values of the variables have to be converted into dimensionless codified values. With such a purpose, the following equation was used
where is the coded value of theith independent variable, is the natural value of such variable, is the natural value at the center point andS is the value of the step change.
The experimental results were statistically analyzed and the results validated by means of an analysis of variance (ANOVA) procedure performed at a confidence level of 95 %. The values of the removal efficiencies (in %) of the four parabens after a contact time of 48 h were selected to perform the statistical analysis. It was calculated as follows
where represents the concentration of each of the parabens initially present in solution and is the final concentration of pollutant determined once the contact time (i.e., 48 h) has elapsed.
As indicated above, the effect of initial concentrations of HO and Fe was analyzed by applying response surface methodology (RSM). Table 1 shows the operational conditions of the statistical design of experiments (DoE), whereas Table 2 summarizes the experimental arrangement behind the DoE and the response obtained in each of the experiments.
Results and discussion
Numerical analysis: analysis of variance report
The analysis of variance test indicates if a given parameter exerts a significant influence on the response variable. Such analysis is summarized in Table 3. The results shown in this Table indicate that, in all cases, the [] and [Fe] exhibit ap value below 0.05, which means that such factors significantly affect the response variable (Yparabens %) at a confidence level equal to 95 %. The same applies for the square of [HO] excepting for the removal of MP (i.e.,p value = 0.0513).
Non-linear polynomial regression was carried out and a equation similar to Eq. 10 was proposed for each of the pollutants. The values of the fitting coefficients obtained for the removal of the four parabens are summarized in Table 4.
From the data listed in Table 4 it may be concluded that, in all cases, [HO] and [Fe] exhibit a positive (+) sign. This means that both positively affect the response variable. Thus, the larger [HO] and [Fe], the more effective is the removal efficiency. The remaining factors are behind a negative (−) sign in Table 4. This latter indicates that such factors negatively influence the removal process. Hence, as these factors increase, the removal of the pollutants is hindered. The factors that exert a negative effect on the removal of parabens are the combination of hydrogen peroxide concentration and Fe ion concentration; [HO] and [Fe] .
The correlation factors, , corresponding to the mentioned equations are 79.2, 78.6, 77.4 and 78.6 %, respectively, which suggests that the proposed model is adequate to describe the experimental results in a reasonable manner. This will be confirmed by the graphical analysis (see next section).
Graphical analysis
The experimental results were modelized according to five factors by using the general Eq. 10 and its particularized form for each of the parabens under study (see Table 4).
In this section, six different kinds of plots will be presented (i.e., Pareto plot, main effect, interaction between variables, response surface, contour and observed vs. predicted plots).
The Pareto plot (Fig. 2) graphically represents the results of the ANOVA test. A bar is plotted to depict the standardized effects of the different factors that are included in the analysis, namely, [HO] (A) and [Fe] (B), and all their possible combinations (i.e.,AA, BB andAB). Gray bars represent the positively affecting factors, whereas blue bars correspond to factors negatively affecting the removal of parabens. As expected, the former appear behind a (+) sign in Table 4 and the later follow a (−) in such table. It is also worth noting the occurrence of a vertical rule at a value around 2. This vertical rule determines the significance level of the ANOVA test at 95 % confidence. Hence, factors reaching this line affect the removal of the different parabens in a significant manner from the statistical standpoint and exhibit ap value below 0.05 in Table 3. The remaining factors do not affect the response variable in a statistically significant manner and exibitp values above 0.05 in the ANOVA test.
The main effect plots (see Fig. 3) illustrate the result of altering one of the variables (A orB), maintaining the other one constant at its central value. In all cases the trend to reach a maximum is clearly pointed out. For both variables such a maximum is achieved whenA orB reach values close to 1. This fact suggests that finding an optimum for the removal of each of the pollutants within the working range is feasible.
The response surface plot is perhaps the most interesting graph when an RSM analysis is performed. It is a plot of Eq. 10 and makes it possible to evaluate the evolution of the system in a qualitative manner. The response surface plots corresponding to the four pollutants here studied are depicted in Fig. 4. Contour plots are presented under their respective response surfaces. It can be appreciated that, in all cases, the response plot corresponds to a convex surface within the whole working interval (i.e., from −1 up to +1). Both variables, (namely, [HO] and [Fe]) affect the removal efficiency in a similar manner and—as suggested by the main effect plots—it is possible to optimize the removal of each paraben. It has to be taken into consideration that if a maximum is observed in the response surface and/or contour plot, it may be stated that under these particular operational conditions an optimum is achieved. Table 5 summarizes the coded and natural values of each of the variables that define an optimum for each pollutant. In all cases the optimum can be found for positive coded values of [HO] and [Fe].
Finally, the observed vs. predicted plot (Fig. 5) provides information on the goodness of the fitting of the experimental results to the proposed model. It can be appreciated that the model is able to predict the experimental results reasonably well.
Physical meaning of the results
It has already been shown that [HO] is the factor that influences the response variable in a more remarkable manner. A possible explanation to this fact can be deducted from Eq. 1. The hydrogen peroxide decomposition generates the hydroxyl radical. This process is catalyzed by Fe in the so-called Fenton process. Remarkably, however, an optimum for the hydrogen peroxide concentration can be appreciated in the main effects plots at values of this factor very close to one. Particularly, the coded and natural values of [HO] that provide an optimal removal efficiency of the four pollutants can be seen in Table 5. This is foreseeable since an increase in [HO] may promote radicals inactivation (or “scavenging”, see Eq. 1). This results in the increase of the concentration of the hydroperoxyl radical, a chemical species with a remarkably smaller value of the oxidation potential [20, 24]. Ferrous ion initial concentration is the second variable in importance. According to the modelization here reported, an optimal removal of the pollutants can be achieved under the operational conditions summarized in Table 5.
Experimental confirmation of the optimum
Four experiments were performed by operating under the theoretically optimal conditions predicted by the model with the aim of determining the presence of a maximum in the removal of the diferent parabens. In all cases, the removal efficiencies of all of the parabens reached nearly 100 %. These results clearly point out that the DOE and RSM strategies here reported are appropriate to analyze the removal of the four pollutants from water by the Fenton process.
Experimental confirmation of the maximum in other aqueous matrices
Two series of experiments were carried out operating at the optimal values of [] and [Fe] for the removal of the four pollutants. With the aim of analyzing the removal of parabens in real waters, two diferent aqueous matrices, namely river water and swamp water, were selected. For comparison with the experiments performed in Milli-Q™ water, a reaction time of 48 h was selected. Under these experimental conditions, removal efficiencies of parabens were determined reaching values very close to 50 % in river water. On the contrary, conversions ranging from 13 up to 18 % were attained in swamp water.
Optimization of the simultaneous removal of all pollutants
To optimize multiple responses (in this case the simultaneous removal of all four pollutants) the use of the so-called desirability function approach is commonly accepted. This procedure is able to determine the operational conditions that give rise to the responses that maximize the desirability function.
Briefly, according to Khasawneh et al. [25] for each response (x), a desirability function () assigns numbers between 0 and 1 to the possible values of , with () = 0 representing a completely undesirable value of and () = 1 representing a completely desirable or ideal response value. The individual desirabilities are then combined using the geometric mean, which gives the overall desirabilityD:
withz denoting the number of responses. Notice that if any responseY is completely undesirable d(Y ) = 0, then the overall desirability is zero. The desirability function can adopt different expressions if a given responseY has to reach a maximum, a minimum or a specific value.
The desirability function corresponding to the process under analysis is depicted in Fig. 6. It can be easily concluded that it is possible to attain a value ofD very similar to 1 for coded values of [HO] and [Fe] in the close vicinity of 1. This suggests that the optimization of the simultaneous oxidation of the four pollutants is feasible under these experimental conditions. Table 5 also summarizes the coded and real values of [HO] and [Fe] that yield to a maximum simultaneous removal of the four pollutants. Such values are 2.6210 and 2.6210M, respectively. Operating under these conditions, at least theoretically, removal percentages of 93.1, 97.3, 97.1 and 100 % can be achieved for MP, EP, PP and BP, respectively.
To corroborate these theoretical maxima, an experiment has been performed in Milli-Q water under the above-referred conditions. The removal percentages for the aforesaid pollutants were 93.0, 97.1, 96.9 and 99.8, respectively.
Conclusions
In this work, the removal of four parabens from water by means of Fenton oxidation was optimized with the application of a central, composite, orthogonal and rotatable design of experiments. From the analysis of the experimental data, the following conclusions may be drawn:
-
The initial concentration of HO exerts the most significant effect on the response variable, followed by [Fe].
-
According to the analysis of variance test, three factors (namely [HO], [Fe] and the squared [HO]) exert a significant influence on the removal efficiency of the pollutants. A second-order polynomial equation has been satisfactorily used to determine the removal efficiency of each chemical. Correlation factors comprising between 0.77 and 0.79 were obtained.
-
Response surface methodology made it possible to optimize the degradation of the pollutants. When the adequate operation conditions were applied, an almost complete removal of the parabens was achieved.
-
Simultaneous optimization of the removal of the four pollutants was achieved, with removal efficiencies ranging 93.0–99.8 % operating with initial concentrations of hydrogen peroxide and Fe ion equal to 2.6210and 2.6210M, respectively.
-
In Fenton process, after a reaction time of 48 h, the removal of parabens reached values of conversions efficiency between 85 and 94 % in Milli-Q water. For river water and swamp water, such parameters reached 50 % and 13–18 %, respectively.
Abbreviations
- :
-
Offset term
- :
-
Linear effect
- :
-
First-order interaction effect
- :
-
Squared effect
- BP:
-
Buthylparaben
- :
-
Initial concentration of pollutant in solution
- :
-
Final concentration of pollutant in solution
- D :
-
Value of the overall desirability function
- ():
-
Value of the individual desirability function
- EP:
-
Ethylparaben
- k :
-
Number of independent variables or factors
- MP:
-
Methylparaben
- n :
-
Number or replicates of the central experiment
- N :
-
Number or runs in the statisitical design of experiments
- PP:
-
Propylparaben
- S :
-
Step change
- :
-
Natural value of theith independent variable at the central point
- :
-
Coded value of theith independent variable
- :
-
Natural value of theith independent variable
- :
-
Predicted response (removal percentage)
- z :
-
Number or responses in desirability function
References
Halling-Sorensen, B., Nors Nielsen, S., Lanzky, P., Ingerslev, F., Jorgensen, S.: Occurrence, fate and effects of pharmaceutical substances in the environment: a review. Chemosphere 36(2), 357–393 (1998)
Jones, O., Voulvoulis, N., Lester, J.: Human pharmaceuticals in the aquatic environment a review. Environ. Technol. 22(12), 1383–1394 (2001)
Calamari, D., Zuccato, E., Castiglioni, S., Bagnati, R., Fanelli, R.: Strategic survey of therapeutic drugs in the rivers Po and lambro in Northern Italy. Environ. Sci. Technol. 37(7), 1241–1248 (2003)
Ternes, T.: Emerging substances in water. In: Hanke, G. (ed.) Workshop on Emerging Environmental Pollutants. Key Issues and Challenges, Stresa. European Commission. DG Joint Research Centre. Institute for Environment and Sustainability (IES), Torino pp. 10–11 (2006)
Barr, L., Metaxas, G., Harbach, C., Savoy, L., Darbre, P.: Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J. Appl. Toxicol. 32(3), 219–232 (2012)
Harvey, P., Everett, D.: Parabens detection in different zones of the human breast: Consideration of source and implications of findings. J. Appl. Toxicol. 32(5), 305–309 (2012)
Calafat, A., Ye, X., Wong, L.Y., Bishop, A., Needham, L.: Urinary concentrations of four parabens in the U.S. Population: NHANES 2005–2006. Environ. Health Perspect. 118(5), 679–685 (2010)
Janjua, N., Frederiksen, H., Skakkebæk, N., Wulf, H., Andersson, A.M.: Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int. J. Androl. 31(2), 118–129 (2008)
Schlumpf, M., Kypke, K., Wittassek, M., Angerer, J., Mascher, H., Mascher, D., Vökt, C., Birchler, M., Lichtensteiger, W.: Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere 81(10), 1171–1183 (2010)
Janjua, N., Mortensen, G., Andersson, A.M., Kongshoj, B., Skakkebæk, N., Wulf, H.: Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ. Sci. Technol. 41(15), 5564–5570 (2007)
Meeker, J., Yang, T., Ye, X., Calafat, A., Hauser, R.: Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ. Health Perspect. 119(2), 252–257 (2011)
Frederiksen, H., Jørgensen, N., Andersson, A.M.: Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). J. Expo. Sci. Environ. Epidemiol. 21(3), 262–271 (2011)
Shen, H., Zhao, Y., Hu, M., Xia, Q.: Preparation and adsorption properties of polycarboxylate nano Fe3O4 magnetic composite particles. Fuhe Cailiao Xuebao/Acta Materiae Compositae Sinica 26(4), 68–73 (2009)
Chin, Y., Mohamad, S., Abas, M.: Removal of parabens from aqueous solution using β-cyclodextrin cross-linked polymer. Int. J. Mol. Sci. 11(9), 3459–3471 (2010)
Perez-Gonzalez, D., Gomez, J., Beristain-Cardoso, R.: Biological removal of p-cresol, phenol, p-hydroxybenzoate and ammonium using a nitrifying continuous-flow reactor. Bioresour. Technol. 120, 194–198 (2012)
Tay, K., Rahman, N., Bin Abas, M.: Removal of selected endocrine disrupting chemicals and personal care products in surface waters and secondary wastewater by ozonation. Water Environ. Res. 83(8), 684–691 (2011)
Hansen, K., Andersen, H., Ledin, A.: Ozonation of estrogenic chemicals in biologically treated sewage. Water Sci. Technol. 62(3), 649–657 (2010)
Hansen, K.; Andersen, H.: Energy effectiveness of direct UV and UV/Htreatment of estrogenic chemicals in biologically treated sewage. Int. J. Photoenergy 1–19 (2012)
de Heredia, J., Domínguez, J., López, R.: Advanced oxidation of cork-processing wastewater using Fenton’s reagent: Kinetics and stoichiometry. J. Chem. Technol. Biotechnol. 79(4), 407–412 (2004)
Domínguez, J., González, T., Palo, P., Cuerda-Correa, E.: Fenton + Fenton-like integrated process for carbamazepine degradation: optimizing the system. Ind. Eng. Chem. Res. 51(6), 2531–2538 (2012)
Lee, H., Shoda, M.: Removal of COD and color from livestock wastewater by the Fenton method. J. Hazard. Mater. 153(3), 1314–1319 (2008)
Wang, S.: A Comparative study of Fenton and Fenton-like reaction kinetics in decolourisation of wastewater. Dyes Pigment. 76(3), 714–720 (2008)
González, T., Domínguez, J., Palo, P., Sánchez-Martín, J., Cuerda-Correa, E.: Development and optimization of the BDD-electrochemical oxidation of the antibiotic trimethoprim in aqueous solution. Desalination 280(1–3), 197–202 (2011)
Arslan-Alaton, I., Tureli, G., Olmez-Hanci, T.: Treatment of azo dye production wastewaters using Photo-Fenton-like advanced oxidation processes: optimization by response surface methodology. J. Photochem. Photobiol. A Chem. 202(2–3), 142–153 (2009)
Khasawneh, M.; Bowling, S.; Kaewkuekool, S.; Cho, B.: A cost effective strength-stress reliability modeling and optimization in engineering design. In: Proceedings of the Industrial Engineering Research Conference, Houston, pp. 323–328 (2002)
Acknowledgments
Financial support from the Spanish CICyT through research projects CTQ- 2010-14823 and CT-2007-60285/PPQ is gratefully acknowledged. MJM and PP wish to thank the Spanish CICyT for their respective Ph.D. research grants.
Conflict of interest
The authors declare that they have no competing interests.
Authors' contributions
JRD carried out the experimental design, including the selection of the variables and their operational ranges. MJM and PP performed most of the experimental work at the lab as part of their respective Ph.D. Theses. JAP performed the preliminary runs that made it possible to determine the starting point of the optimization process. TG and EMCC carried out the statisitical analysis and the discussion of results.
Author information
Authors and Affiliations
Corresponding author
Additional information
5th International Congress on Energy and Environmental Engineering and Management (CIIEM).
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Domínguez, J.R., Muñoz, M.J., Palo, P. et al. Fenton advanced oxidation of emerging pollutants: parabens. Int J Energy Environ Eng 5, 89 (2014). https://doi.org/10.1007/s40095-014-0089-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40095-014-0089-1