Abstract
In this study, the effect of oxygen content on a thin copper oxide layer deposited on BK7 and steel substrates by DC magnetron sputtering were investigated. Argon as working gas with impurity of 99.9% and various oxygen ratios were used to sputter a pure Cu cathode target in a cylindrical geometry. The produced samples were analyzed by X-ray diffraction (XRD), energy-dispersive X-ray (EDX), atomic force microscopy (AFM), and spectrophotometry techniques. The films thickness was measured by profilometer facility. The results show that by increasing oxygen content in the working gas the sputtering rate reduces. Moreover, the type of oxide phase (Cu2O or CuO) in the synthesized layer and consequently its optical properties dramatically depend on Ar/O2 ratio in the working gas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper oxide as a thin film is an attractive material for various applications such as semiconductors, sensors, smart windows and photovoltaic devices [1,2,3]. Low-cost production abundant availability and nontoxic nature are some attractive features of this material. Thus, investigation of copper oxide films has both scientific and technological significance. It is important to know that the preparation technique can define the electrical and optical properties of the films [4]. Various methods such as evaporation [5,6,7], chemical vapor deposition [8], sputtering [9,10,11,12,13], thermal oxidation [14], sol–gel process [15], were used to produce the copper oxide film. The advantages of sputtering method are its flexibility, scalability, complexity of possible films, reproducibility, ability to work at low temperature, capability to deposit precisely controlled hetero structures, and simplicity of controlling the film composition, structure and morphology. The DC magnetron sputtering technique in the reactive mode is one of the ways to synthesize copper oxide thin films due to the excellent uniformity and controllability.
In this paper, the role of oxygen content added to the argon as working gas (WG) on the structural, compositional and optical properties of copper oxide film during magnetron sputtering process are reported. The use of a premixed WG (Ar + O2), cylindrical geometry of the magnetron sputtering system, simultaneous applying of two substrates (i.e., steel and transparent Bk7 glass), and investigation of morphology and thickness alongside other properties of the deposited films are some of the new features of the current research.
Experimental details
The polished AISI316 stainless steel and transparent Bk7 substrates (1 × 1 cm2) were cleaned with acetone, ethyl alcohol, and de-ionized water in an ultrasonic bath, for 10 min each. DC magnetron sputtering system is schematically shown in Fig. 1. In this geometry, cathode (inner one, made from copper with impurity ~ 99.99% and 3 cm diameter), and anode (outer one, made from aluminum) are two cylinders.
WG in the deposition chamber was pure Ar (99.99%) or premixed Ar + O2 with 5, 10, and 20% oxygen. Films were deposited under a fixed condition are presented in Table 1.
The sputtering process for BK7 and steel substrates were simultaneously performed for each sputtering regime. Transparent substrate (BK7) was used to obtain optical properties (i.e. transmission). The films thickness was measured after deposition process with a Surface Profile Meter, Bruker Dektak XT profilometer. To measure the standard error, the thickness of the deposited layer obtained at seven different points of the samples. The structure of the films were characterized by X-ray diffraction using a STOE model STADI MP diffractometer which uses Cu kα (λ = 0.154 nm) radiation. The surface morphologies were observed by Atomic Force Microscopy, Park Scientific Instrument, Auto probe CP. The optical transmission and reflection measurements in the range of 175–2500 nm were performed using a UV–Vis–NIR Spectrophotometer, Varian, Cary 500. Energy-dispersive X-ray (EDX) spectroscopy was carried out with AMETEK EDAX analyzer.
Results and discussion
Film thickness
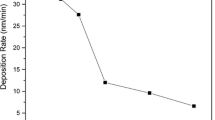
The thickness of deposited layer and its evolution by increasing the oxygen content in the WG is shown Fig. 2. In the case of using Ar and Ar + 5% O2 no remarkable difference is observed. Since the deposition time for all samples was constant, it can be concluded that 5% oxygen in the WG does not significantly change the sputtering yield and consequently the deposition rate. But there is a significant decrease (by a factor of two) in the thickness of sputtered layer for WGs with higher oxygen content (10 and 20%). This reduction of thickness at higher oxygen content reflects the less efficiency of sputtering by oxygen plasma when compared with Ar plasma. It may also be possible that more oxygen in the WG causes the oxidation of the Cu target and oxidized target has lower sputtering rate.
XRD and EDX results
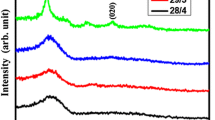
Figure 3 shows the XRD patterns of the samples before and after film deposition on steel substrates. The three peaks of the γ-Fe for the nickel–chrome steel is seen. When Ar is used as the WG of the sputtering process, the diffraction peaks at 2θ = 43.50° and 2θ = 50.90° enhance. Since the diffraction peaks of Cu(111) and Cu(200) are at the same position of γ-Fe, this increase in the intensity is the evidence of the crystalline copper layer without any oxide phase. The injection of 5% of oxygen in the WG leads to appearance of a small new peak at 2θ = 37.06°. According to PDF card #34-1354, this peak is related to the cubic phase of Cu2O (111). As seen in Fig. 3, this oxide phase at higher oxygen content is converted to the enriched oxide phase as CuO. Using Ar + 10% O2 as WG, at 2θ = 35.38° and according to PDF card #45-937, CuO (002) has been formed. Using Ar + 20% O2 as WG, the same peak exists. According to the results of the thickness measurements, the decrease in the intensity of the CuO peaks at Ar + 20% O2 is connected to the oxidation of the Cu target or reduction of the sputtering rate and amount of the deposited layer.
For investigating the effect of substrate on phase composition of deposited layer, the XRD pattern of sample with BK7 substrate and Ar + 5% O2 as WG is demonstrated in Fig. 4. The hump for 2θ < 35° is related to the amorphous state of the substrate. Similar to steel substrate this result confirms the formation of Cu2O and Cu phases.
To obtain the atomic concentration of surface, the EDX analysis was performed. Table 1, shows the EDX results of two samples in a condition with 5 and 20% oxygen in the WG. According to Table 2, adding oxygen to WG, the deposited layer is saturated by oxygen. In the 20% case, this saturation is significant to expect the formation of a single phase of enriched oxygen (i.e. CuO). This result is in agreement with XRD study.
Therefore, the controlling the phase composition in the deposited layer is possible by controlling the amount of O2 in the WG of the sputtering process.
AFM results
Figure 5 shows the AFM images of samples with or without oxygen in the WG of the sputtering process. The substrate for all images was steel. Figure 5.a represents the AFM result of the sputtered film when pure argon gas is utilized for plasma generation. Figure 5.a shows that multi-level configurations (submicron structures with ~ 0.1 µm in size consist of spherical nano-scale structures with ~ 10–20 nm in radius) are observed on the surface. Adding 5% oxygen gas in the WG of the sputtering process the structure of the resulted films significantly changes as seen in Fig. 5b. The first level (smaller nano-sized structures) disappears and the single enlarged grains are formed. Moreover, these grains become smaller at higher oxygen ratios (see Fig. 5b–d).
Although it is a difficult task to find a relationship between structures of the surface and amount of O2 in the WG, but it can be connected to the creation of the new compositional phase (Cu, Cu2O or CuO). Comparing Fig. 5b with c and d, it can be suggested that the decrease in the grain size at higher oxygen ratios (10 and 20%) may be related to the smaller crystallites of CuO phase to Cu2O phase.
Therefore, the presence of O2 and its amount in the WG of the sputtering process not only change the composition of the sputtered film but also change the structure and morphology of the film.
Optical properties
Figure 6a and b shows the optical transmission of the samples (with BK7 substrates) at wavelengths in the range of 200–2500 nm which were prepared under various O2 content. Sputtered thin films without oxygen or with 5% O2 in the WG have a very low transmission (Fig. 6a. According to the XRD analysis, this is due to the presence of non-transparent Cu and Cu2O in the prepared films. With the increase in the amount of O2 in the WG, the transmission deposited films dramatically increase (Fig. 6b. To explain the optical behavior for the thin films sputtered with 10 and 20% oxygen in the WG, the optical band gap energies (Eg) were calculated. A substantial drop in Fig. 6 near the absorption edge is related to the excitation of charge carriers over the optical band gap [11, 16].
The following formula shows that how incident photon energy is related to absorption coefficient (α) [16]
in this relation h is Planck’s constant, ν is the frequency of light, A is the edge width parameter, and value of n depends on the type of transition [16]. Here the value of n is 0.5 due to the direct allowed transition. By plotting (αhν)1/n versus(hν) a straight line is acquired and then optical band gap can be determined by extrapolation of the linear portion of the plots of (αhν)1/n versus (hν) to α = 0. The transmittance (T), reflectance (R) and thickness of the film (d) provide \(\alpha\) by [17]:
Figure 7 shows the (αhν)2 versus hν for the copper sputtered in the WG containing 10 and 20% oxygen. The optical band gap of 2.10 and 2.26 eV obtained, respectively. The results indicate that by enhancing the oxygen content in the WG, the optical band gap increases. The higher band gap energy for deposited layer with 20% oxygen in WG is due to the greater transmittance of visible light as compared to the case of 10%. In contrast to this, for 5 and 0% oxygen in the WG, since metallic Cu is dominant in these samples the explained method cannot be used to determine the optical band gap because metal does not have band gap structure.
Tauc plot [18] of samples for 10 and 20% oxygen content of WG in the sputtering process
Conclusions
Copper oxides (Cu2O and CuO) thin layers were produced by dc magnetron sputtering technique in a mixture of Ar and O2 gases and various oxygen contents. The increase in the amount of oxygen in the WG led to the decrease of the deposition rate and formation of the oxide phases from Cu2O to CuO. The results showed that by increasing the amount of oxygen in the WG, the optical band gap enhanced. The optical band gap energies of 2.10 and 2.26 eV obtained for 10 and 20% oxygen in the WG. Therefore, it can be concluded that by adjusting the amount of oxygen in the WG of the sputtering process the phase composition, optical behavior, and structure of the copper thin films can be controlled.
References
Tao, M., Marson B., Rajeshwar K.: Next Generation Photovoltaics and Photoelectrochemistry Electrochemical Society. ECS Transactions. 11, 9 (2008)
Mittiga, A., Salza, E., Sarto, F., Tucci, M., Vasanthi, R.: Heterojunction solar cell with 2% efficiency based on a Cu2O substrate. Appl. Phys. Lett. 88, 163502 (2006)
Akimoto, K., Ishizuka, S., Yanagita, M., Nawa, Y., Paul, G.K., Sakurai, T.: Thin film deposition of Cu2O and application for solar cells. Sol. Energy. 80, 715 (2006)
Derin, H., Kantarli, K.: Optical characterization of thin thermal oxide films on copper by ellipsometry. Appl. Phys. A. 75, 391 (2002)
Balamurugan, B., Mehta, B.R.: Optical and structural properties of nanocrystalline copper oxide thin films prepared by activated reactive evaporation. Thin Solid Films 396, 90 (2001)
Al-Kuhaili, M.F.: Characterization of copper oxide thin films deposited by the thermal evaporation of cuprous oxide (Cu2O). Vacuum. 82, 623 (2008)
Tounsi, N., Barhoumi, A., Chaffar Akkari, F., Kanzari, M., Guermazi, H., Guermazi, S.: Structural and optical characterization of copper oxide composite thin films elaborated by GLAD technique. Vacuum. 121, 9 (2015)
Maruyama, T.: Copper oxide thin films prepared by chemical vapor deposition from copper dipivaloylmethanate. Solar Energy Materials and Solar Cells. 56, 85 (1998)
Lee, H.H., Lee, C., Kuo, Y.L., Yen, Y.W.: A novel two-step MOCVD for producing thin copper films with a mixture of ethyl alcohol and water as the additive. Thin Solid Films. 498, 43 (2006)
Reddy, A.S., Uthanna, S., Reddy, P.S.: Properties of dc magnetron sputtered Cu2O films prepared at different sputtering pressures. Appl. Surf. Sci. 253, 5287 (2007)
Reddy, A.S., Park, H.H., Reddy, V.S., Reddy, K.V.S., Sarma, N.S., Kaleemulla, S., Uthanna, S., Reddy, P.S.: Effect of sputtering power on the physical properties of dc magnetron sputtered copper oxide thin films. Mater. Chem. Phys. 110, 397 (2008)
Reddy, A.S., Reddy, P.S., Uthanna, S., Rao, G.V., Klein, A.: Effect of substrate temperature on the physical properties of dc magnetron sputtered Cu2O films. Phys. Status. Solidi. A. 203, 844 (2006)
Reddy, A.S., Rao, G.V., Uthanna, S., Reddy, P.S.: Influence of substrate bias voltage on the properties of magnetron sputtered Cu2O films. Phys. B. 370, 29 (2005)
Hsieh, J.H., Kuo, P.W., Peng, K.C., Liu, S.J., Hsueh, J.D., Chang, S.C.: Opto-electronic properties of sputter-deposited Cu2O films treated with rapid thermal annealing. Thin Solid Films. 516, 5449 (2008)
Musa, A.O., Akomolafe, T., Carter, M.J.: Production of cuprous oxide, a solar cell material, by thermal oxidation and a study of its physical and electrical properties. Sol. Energy. Mater. Sol. Cells. 51, 305 (1998)
Ray, S.C.: Preparation of copper oxide thin film by the sol–gel-like dip technique and study of their structural and optical properties. Sol. Energy Mater. Sol. Cells. 68, 307 (2001)
Fox, M.: Optical properties of solids. Oxford University Press, New York (2001)
Tauc, J.: Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 3(1), 37–46 (1968)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Aghilizadeh, N., Sari, A.H. & Dorranian, D. Role of Ar/O2 mixture on structural, compositional and optical properties of thin copper oxide films deposited by DC magnetron sputtering. J Theor Appl Phys 11, 285–290 (2017). https://doi.org/10.1007/s40094-017-0268-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40094-017-0268-6