Abstract
Purpose
The effect of different initial carbon to nitrogen (C/N) ratios on the prevalence of Salmonella spp., fecal coliforms and helminth eggs over composts produced from several mixtures of maize straw (S) and dairy manure (M) was investigated.
Methods
Four C/N ratios (21, 22, 27, and 38) were evaluated, including one with manure only (C/N 21). The composting process was performed under field conditions in northern Mexico.
Results
The process lasted 51 days; Salmonella spp. was reduced 1–2 log (> 94%) in most treatments, except for the C/N ratio of 27 which achieved < 1 log reduction (about 35%). Fecal coliforms elimination was 3–4 log (> 99%) in all treatments while helminth eggs achieved < 1 log (72–87%, depending on treatment). In this study, the mixture with initial C/N ratio of 22 (25% S + 75% M), which had the lowest amount of straw, resulted in the highest elimination of Salmonella spp., fecal coliforms, and helminth eggs. This mix complied with current Mexican sanitary regulations for compost use. The composts produced from the other C/N ratios complied only with the limits for one or two of the microorganisms that were analyzed.
Conclusions
The initial C/N ratios in compost from straw and manure influences microbial reduction. The final C/N of the mixes ranged from 14 to 16, indicative of stable compost. Compared to fecal coliforms, Salmonella spp. and helminth eggs were more resilient.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Concentrated animal feeding operations (CAFOs), each defined as a farm operation which has over 1000 animal units confined for over 45 days a year (USEPA 2001), produce large amounts of waste mainly in the form of manure. In northern Mexico, the area known as “La Laguna” (25°33′00″ N, 103°26′00″ W) is home to almost 600,000 cows (SAGARPA 2016) distributed in both CAFOs and smaller farms which produce milk and beef meat. According to Nennich et al. (2005), a lactating cow produces 9.7 kg day−1 of dry manure. Assuming that 75% of the heard size is in the production stage, the amount of solid waste generated in the area is estimated at 5450 ton day−1 (nearly 2.0 million ton year−1) of dry manure.
Among the biological processes available for organic waste treatment, such as manure, are anaerobic digestion (AD) and composting. AD requires the use of vessels or containers with an impermeable cover to avoid oxygen to get into the reactors. AD usually also requires more monitoring and maintenance (e.g. leaks detection and use of the biogas produced) for proper operation. On the other hand, composting is an effective means of recycling organic waste for agricultural use and an accepted waste treatment method (Raj and Antil 2011). In addition to manure, materials often used as substrates for composting include yard waste, the organic fraction of municipal solid waste, other wastes such as animal carcasses (Shepherd et al. 2010), and/or combinations of them. Typical disposal methods for animal manure in Mexico include the application of raw (untreated) manure on agricultural land and, to a lesser extent, composting. Direct application of raw manure to farmlands has the potential to create human and animal health risks and cause contamination of surface and groundwater (Ogunwande et al. 2008) and the spread of manure pathogens is also of concern (Millner et al. 2014). Composting is a feasible alternative to minimize the potential adverse effects on the environment and public health related to disposal of raw manure on land and agricultural fields.
A successful composting process depends primarily on the adequate control of variables such as temperature, moisture content, aeration, pH, carbon to nitrogen (C/N) ratio, and feedstock mixtures (Gao et al. 2010). The optimum conditions of the compost pile include temperature 55–60 °C (during the thermophilic stage), moisture content in the mixture 40–60%, pH 5.0–8.0, and initial C/N ratios between 20 and 40 (Neklyudov et al. 2008). However, Vochozka et al. (2017) mention that international technical standards require a C/N range of 20–30. Dadi et al. (2019) have identified the C/N ratio as one of the major factors affecting the quality of compost. High ratios make the process very slow as there is an excess of degradable carbon for the microorganisms, but low C/N ratios indicate an excess of nitrogen which may be lost from the process (Antil et al. 2014). Therefore, inadequate C/N ratios may need to be corrected by adding another material to get a balanced content of both elements. Relative proportions of manure and carbon amendments in compost may also affect the metabolic response of indigenous compost microflora and, in consequence, the survival of zoonotic pathogens (Erickson et al. 2009). Another reason why the C/N balance is important in composting is the optimization of bulking materials which in some areas may be of limited availability.
The microorganisms needed for composting are found in compost feedstocks, maintaining an active microbial population during the process (Karnchanawong and Nissaikla 2014). However, sanitation and biosecurity issues related to the safe use of composts are a concern. Although indicator bacteria do not necessarily cause illness, they are abundant in human waste where other organisms such as pathogenic bacteria, viruses, and parasites (e.g. helminth eggs) are also likely to exist. Among the most frequently used indicators of fecal contamination are total coliforms (TCs), fecal coliforms (FC), and enterococci (Noble et al. 2004). However, due to the cost of monitoring equipment and compost analyses, the process may not be adequately monitored or controlled. For instance, in Mexico composting practices are often carried out by small farmers and at family-size facilities which lack of mechanical and process-control equipment. Therefore, it is often performed using static piles which are passively or manually aerated by turning and mixing the materials periodically with the use of shovels or other rudimentary instruments. Consequently, the quality and sanitation of the final product may remain unknown and a concern to final users. With the purpose of guaranteeing the hygienic safety of composts, many countries have defined quality criteria (Brochier et al. 2012). The most common criteria for pathogen presence are Salmonella and E. coli; helminth ova is another parameter included in some European Member States regulations (Cesaro et al. 2015). Examples of regulations applicable to biosolids (i.e., sludge and solids generated from wastewater treatment processes) and compost are presented in Table 1. In current Mexican regulations, fecal coliforms content (not E. coli) is used as the indicator of fecal contamination.
As previously mentioned, the C/N ratio is a key factor in compost production. The effect of C/N ratio on decomposition after amendment with individual types of waste and their mixes has been studied extensively, but less is known about the effect of highly decomposed organic materials such as manures on interactions in mixes (Truong and Marschner 2018). The C/N ratio has shown to influence pathogen survival during composting (Chen et al. 2018). Millner et al. (2014) found that the use of straw to increase aeration, self-heating capacity, and heat retention in manure piles enhanced pathogen die-off and reduced the risk of environmental spread when manure is applied to land. However, their work was based on a single C/N ratio of 23. The objectives of this research were: (1) to evaluate the effect of the initial C/N ratio on the occurrence of Salmonella spp., fecal coliforms, and helminth eggs in composts produced from different mixtures of dairy manure (M) and maize straw (S); (2) to determine the conditions (most effective C/N ratio) under which the compost that was produced complied with current sanitary conditions for its use and sale in Mexico.
Materials and methods
Materials
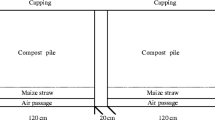
The organic wastes used in this research, dairy manure (M) and maize straw (S), are available in large quantities in La Laguna. Manure (M) was collected from feedlot piles (< 6 months old) from a local dairy farm and transported to the research site located in northern Mexico (25°35′ N, 103°27′ W; 1120 m above mean sea level). As a general practice in arid regions, manure is typically removed from open feedlots every 6 months for final disposal. Thus, this research used manure of the same age. Straw (S) was obtained from a local farm and chopped to particles < 10 cm long. The composting process was performed under field conditions using static piles manually aerated.
Composting
The experimental design consisted of randomized blocks with four treatments (Table 2) and four replicates each. Initially, the experimental static piles consisted of 1.2 m × 0.8 m plots, about 0.40–0.45 m high, depending on the type of mixture. Aeration was achieved by periodically mixing the piles using a shovel to promote aeration and homogeneity. A recent study conducted at a small composting operation also used static piles of about the same size as in this study, but air was supplied through a perforated pipe and a blower (Omrani and Samavat 2017). Temperature in the compost piles was measured and recorded every other day at a depth of 25–30 cm at three different points: (1) top of the pile; (2) lateral at one side of the pile; and, (3) center of the pile (inside the pile after removing some material from a side to get to the center). Compost temperatures were averaged to include the three sampling points in each pile and the replicates of each treatment.
Sampling
Samples were collected following the procedures established in the manual Recommended Methods of Manure Analysis (Peters et al. 2003) and Mexican regulation NOM-004-SEMARNAT-2002 (SEMARNAT 2002). This regulation is based on some of the analytical methods established by the United States Environmental Protection Agency (EPA) and the Standard Methods for the Examination of Water and Wastewater (authored by APHA, AWWA, WPCF. 1992, 18th edn), among other international analytical protocols. Samples were taken at four different stages of the composting process: (1) day 1 (initial); (2) day 15 (thermophilic phase); (3) day 30 (about the middle of the process); and, (4) day 50 (final product). At each sampling time, four sub-samples were collected from different parts and depths of the pile and mixed thoroughly into a single composite sample. The samples were refrigerated at 4 °C until microbiological analyses were performed (< 72 h after collection). To minimize variability, bias, and error, the same person collected and analyzed all the samples through the duration of the research.
Physicochemical analyses
Total solids (TS) and moisture content were determined by oven-drying 50 ml of sample at 70 °C until constant weight was attained (Peters et al. 2003). For pH and electrical conductivity (EC) determinations (Thermo Electron, Corp. pH and EC meters, respectively), a water extract was prepared with the as-received sample by adding one part of solid sample to three parts of distilled water (v/v). To calculate the C/N ratio, organic carbon (OC) was determined in a muffle furnace through the loss on ignition (LOI) method at 550 °C for 2 h. Total Kjeldahl nitrogen (TKN) was analyzed by distillation followed by titration with sulfuric acid and nitrate content was determined by distillation.
Microbiological analyses
For Salmonella spp. and fecal coliforms analyses, 36 ml of distilled water were added to the equivalent of 4 g TS. Samples were incubated at 37 °C for 24 h. Successive dilutions were performed to a final 1/10,000. All samples were analyzed in triplicate and enumerated by the three-tube most probable number (MPN) procedure according to Mexican federal regulation NOM-004-SEMARNAT-2002 (SEMARNAT 2002).
Salmonella spp.
For the quantitative analysis of Salmonella spp., test portions of the serial dilution previously described were inoculated into tubes containing selenite-cystine broth and incubated at 41 °C for 24 h. Samples taken from positive tubes were spread onto brilliant green and Salmonella–Shigella (SS) agar plates. After incubation, colonies that were suspected positive were streaked on triple sugar iron (TSI) and lysine iron agar (LIA) plates for biochemical confirmation.
Fecal coliforms
The MPN test was used for fecal coliforms determination. Three-test tubes series were incubated at 44.4 °C for 24 h. Positive tubes where those which showed gas production in the Durham tubes.
Helminth eggs
Quantification of helminth eggs was performed on 2 g of sample (dry weight) following the flotation/settling procedures established in Mexican regulation NOM-004-SEMARNAT-2002 and then observed under the microscope (Carl Zeiss, Mod. Axio Scope A1) using a Sedgwich-Rafter cell (SEMARNAT 2002).
Statistical analysis
The normality of the data was established with the test of Shapiro–Wilks. Analysis of variance (ANOVA) was used to compare the means of Salmonella spp., fecal coliforms, and helminth eggs among treatments considering composting time as variation factor. To determine the overall efficiency of the composting process on the reduction of the three microorganisms, the ANOVA test was conducted taking initial concentrations (day 1) as the base and comparing them to final concentrations. The Tukey test was also conducted at p < 0.05 to test statistically significant differences among treatments and/or composting time. The statistical analysis was carried out using SPSS software (version 21).
Results and discussion
Composting process
At the end of the composting process (51 days), the product showed granular and uniform appearance without distinction between the initial individual components, except for the manure only treatment (T4) where small lumps and aggregates were formed over time. This characteristic shows the importance of using bulking materials to promote aeration among the particles and to improve compost consistency and homogeneity. The color of all the piles was dark brown and had no foul odor. Moisture content varied according to the mixture of substrates. Considering all the piles: initial (t = 0) was 61% average, 75% maximum and 55% minimum. Final was 53% average, 74% maximum, and 43% minimum. Among the environmental parameters of importance for composting, temperature is critical to evaluate process effectiveness and the degree of stabilization (Bueno et al. 2008). Figure 1 shows ambient temperatures which were in the range of 23–30 °C and averaged 28 °C during the composting process. The difference between local ambient temperatures and the composting piles at the beginning of the process (days 1–20) is noticeable. Conventional composting includes a thermophilic phase (45–65 °C) during which labile organic matter is rapidly degraded by thermophilic microflora (Haynes and Zhou 2016). Initial temperatures for T1 and T2 were > 60 °C, compared to slightly lower (> 55 < 60 °C) for T3 and T4; average temperatures through the duration of the process were 48.9 °C (T1), 46.2 °C (T2), 44.8 °C (T3), and 48.2 °C (T4). The decrease in temperature recorded on days 6 and 22 of the process was due to rainfall. However, temperatures increased rapidly after these rain events. The natural fluctuation of the temperature in the compost pile over time shown in Fig. 1 is related to consumption of organics by microorganisms. As the concentration of organic matter and nutrients decreases, there is less active biomass and temperature drops. As shown in Fig. 1, there was a significant rain event on day 45, but it had a minor impact on temperature as most of the organics were consumed by then. The recovery in temperature after rainfall was minor compared to what occurred in previous events early during the composting process.
Compost characteristics
Initial and final values for pH, EC, and C/N ratio are presented in Table 3. All treatments showed higher final pH and EC values compared to the initial. According to some researchers, during the initial stages of the composting process pH tends to be slightly acidic, but towards the end it turns slightly basic due to the changes in humic acids content (Venegas-González et al. 2005). However, in the region where the research was carried out, both groundwater and dairy manure have high pH. Consequently, producing compost with pH near the neutral range is neither common in the region, nor expected in this study. Other studies show the effectiveness of pH management (increase in it) as a modulator of viral reproduction in composts (Auffret et al. 2019). EC also showed an increase in the final product. This may be related to the quality of the water that was added to the compost, which in this region is naturally high in conductivity due to the presence of salts and minerals (alkaline water). Regarding the C/N ratio, the range of values observed among treatments showed a significant decrease from initial 21–38 to final 14–16 (Table 3). This is due to the consumption of organic carbon during composting. Dadi et al. (2019) also reported a decline of C/N with time which indicates that a stable product is formed. Regardless of the initial C/N, final values were similar to the 10–15 reported by Vázquez and Soto (2017), indicative of an advanced process of composting and a good retention of the nitrogen content which favors the conservation of nitrogen as nutrient.
Microbial populations
According to Heck et al. (2013), temperatures reached through the composting process play an important role to effectively reduce pathogen content. As shown in Fig. 1, all mixes showed a thermophilic stage at the beginning of the process, reaching 55–64 °C. In the study by Fournel et al. (2019), with temperatures from 45 to 65 °C, they were effective in the reduction of bacterial growth. In a study conducted by Lafond et al. (2002) it was observed that pathogen survival was closely related to the C/N ratio. Other researchers have reported that in C/N ratios > 20, as in this research, the carbon content is enough to stabilize the nitrogen content, a factor that has an influence on the production of bactericide agents which reduce or eliminate pathogens loads during composting (Park and Diez-Gonzalez 2003).
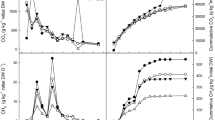
The concentrations of Salmonella spp. through the composting process are shown in Fig. 2 (t = 0 is the initial concentration). The highest concentration was recorded in T3 which had the highest initial C/N ratio (C/N 38). However, the statistical analysis only showed a significant difference (p < 0.05) at the beginning of the process (day 1). The influence of C/N on Salmonella spp. inactivation in cow manure mixtures composted in a bioreactor under controlled conditions was studied by Erickson et al. (2009). They found that for a C/N 20 Salmonella was inactivated in 2.8 days at temperatures < 50 °C, but when the C/N was 40, longer time (3.6 days) and higher temperature (> 55 °C) were needed. The results of this study are comparable to their findings; however, this investigation was performed under field conditions, not in a bioreactor.
Fecal coliforms content is another microbial parameter of importance in compost quality and biosecurity. In this study, significant differences (p < 0.05) were found among the composting times at least in one of the treatments. As presented in Fig. 3, the initial (t = 0) fecal coliforms content was high in all treatments but showed a rapid reduction. Wichuk and McCartney (2007) found that E. coli in compost was eliminated in 25 days at temperatures between 45 and 55 °C; our results for fecal coliforms are comparable to theirs. Other studies reported > 99.9% elimination of fecal coliforms and E. coli within the first 7 days of cattle manure composting between 33.5 and 41.5 °C (Larney et al. 2003). In our study, a significant decrease of fecal coliforms in all treatments was observed for process temperatures 33 to 64 °C, but it took about 30 days to achieve that reduction. Regarding C/N, Lafond et al. (2002) studied the elimination of fecal coliforms and fecal streptococcus in duck excreta enriched with wood shavings. They reported elimination in enclosed composting systems which had initial 32.9 C/N, but only a reduction in concentration in those with initially high C/N (67.5) during the 32 days of their process. In our study, a reduction of fecal coliforms was achieved within 30 days of composting in all treatments for initial C/N ratios between 21 and 38.
The change in helminth eggs content is illustrated in Fig. 4. Except for T2, all other treatments showed significant statistical differences (p < 0.05) from initial to final content of helminth eggs. However, in all cases the first substantial reduction occurred during the first two weeks (thermophilic stage). Wichuk and McCartney (2007) reported that the time–temperature relationship was the key for pathogen elimination. A high temperature for short time can be as effective as a lower temperature for a long period of time. In the case of parasites, such as helminth eggs, Jones and Martin (2003) reported the elimination of Ascaris lumbricoides at 55 °C for 60 min. In the present study, all treatments reached temperatures > 55 °C and remained high for several days (Fig. 1); however, inactivation was not complete. Mensah et al. (2013) mention that helminth eggs have high resistance to chemical and physical conditions such as lime and temperature, and as such, they are the most resistant form of parasites.
Figure 5 shows the total reductions for Salmonella spp., fecal coliforms, and helminth eggs. At the end of the process, Salmonella spp. reduction was > 94% (1–2 log) in most treatments, except T2 which showed a significant difference (p < 0.05) and achieved only 35% (< 1 log) reduction. In this case, one of the samples (replicates) exhibited a high concentration, thus affected the average for the treatment. Except for T1, which complies with current regulations for Salmonella spp., the remaining compost mixtures did not meet the sanitary requirements shown in Table 1 for this pathogen. The final Salmonella spp. concentration in T1 was 3 MPN per 4 g TS which complies with the limits established in Mexican regulations (details provided in Table 1) for composts in the State of Mexico (< 3 MPN per g TS) and Mexico City (< 3 MPN per 4 g TS). Fecal coliforms elimination was highly efficient (> 99%, 3–4 log) in all treatments. Fecal concentration in T1 was 3 MPN per 4 g TS, compared to compost quality in the State of Mexico and Mexico City which is < 1000 MPN per g TS. Regarding helminth eggs, the reductions achieved were between 72 and 87% (< 1 log), being the lowest for T2. However, no significant differences (p > 0.05) were found among treatments. T1 resulted in the lowest amount of helminth eggs and complies with Mexican limits for compost (State of Mexico < 10 per g TS and Mexico City < 1 in 4 g TS). The treatments and time as well as the statistical significance that were effective for bacterial reduction analyzed in this work are presented in Table 4.
Conclusions
The C/N ratio is an important parameter in composting processes as it affects temperature, a key factor to achieve pathogen reduction. Except for one treatment and sampling event (T4, 49 °C on day 6), all compost mixtures attained temperatures > 55 °C during the first eight days of the process. However, this did not result in the elimination of pathogens and indicator bacteria to the limits established in current regulations in all the composts. The initial C/N 22 (T1 = 25% S + 75% M), which initially attained temperature > 60 °C, was the most effective mixture to reduce the concentration of Salmonella spp., fecal coliforms, and helminth eggs. This compost complied with current Mexican sanitary regulations for those microorganisms. Composts prepared from initial C/N ratios of 21, 27 and 38 met at least the limits for one or two of the microorganisms analyzed in this investigation, being helminth eggs the most resilient.
References
Antil RS, Raj D, Abdalla N, Inubushi K (2014) Physical, chemical and biological parameters for compost maturity assessment: a review. In: Maheshwari D (ed) Composting for sustainable agriculture. Sustainable development and diodiversity, vol 3. Springer, Cham, pp 83–101. https://doi.org/10.1007/978-3-319-08004-8_5
Auffret MD, Brassard J, Jones TH, Gagnon N, Gagné MJ, Muehlhauser V, Masse L, Topp E, Talbot G (2019) Impact of seasonal temperature transition, alkalinity and other abiotic factors on the persistence of viruses in swine and dairy manures. Sci Total Environ 1(659):640–648. https://doi.org/10.1016/j.scitotenv.2018.12.306
Brochier V, Gourland P, Kallassy M, Poitrenaud M, Houot S (2012) Occurrence of pathogens in soils and plants in a long-term field study regularly amended with different composts and manure. Agric Ecosyst Environ 160:91–98. https://doi.org/10.1016/j.agee.2011.05.021
Bueno P, Tapias R, López F, Díaz MJ (2008) Optimizing composting parameters for nitrogen conservation in composting. Bioresour Technol 99:5069–5077. https://doi.org/10.1016/j.biortech.2007.08.087
Cesaro A, Belgiorno V, Guida M (2015) Compost from organic solid waste: quality assessment and European regulations for its sustainable use. Resour Conserv Recycl 94:72–79. https://doi.org/10.1016/j.resconrec.2014.11.003
Chen Z, Kim J, Jiang X (2018) Survival of Escherichia coli O157:H7 and Salmonella enterica in animal waste-based composts as influenced by compost type, storage condition and inoculum level. J Appl Microbiol 124:1311–1323. https://doi.org/10.1111/jam.13719
Dadi D, Daba G, Beyene A, Luis P, Van der Bruggen B (2019) Composting and co-composting of coffee husk and pulp with source-separated municipal solid waste: a breakthrough in valorization of coffee waste. Int J Recycl Org Waste Agric 8:263–277. https://doi.org/10.1007/s40093-019-0256-8
Erickson MC, Liao J, Ma L, Jiang X, Doyle MP (2009) Inactivation of Salmonella spp. in cow manure composts formulated to different initial C: N ratios. Bioresour Technol 100:5898–5903. https://doi.org/10.1016/j.biortech.2009.06.083
Fournel S, Godbout S, Ruel P, Fortin A, Duquette-Lozeau K, Létourneau V, Généreux M, Lemieux J, Potvin D, Coté C, Duchaine C, Pellerin D (2019) Production of recycled manure solids for use as bedding in Canadian dairy farms: II. Composting methods. J Dairy Sci 102(2):1847–1865. https://doi.org/10.3168/jds.2018-14967
Gao M, Liang F, Yu A, Li B, Yang L (2010) Evaluation of stability and maturity during forced-aeration composting of chicken manure and sawdust at different C/N ratios. Chemosphere 78:614–619. https://doi.org/10.1016/j.chemosphere.2009.10.056
Haynes RJ, Zhou YF (2016) Comparison of the chemical, physical and microbial properties of composts produced by conventional composting or vermicomposting using the same feedstocks. Environ Sci Pollut Res 23:10763–10772. https://doi.org/10.1007/s11356-016-6197-0
Heck K, De Marco ÉG, Hahn ABB, Kluge M, Spilki FR, Van Der Sand ST (2013) Evaluation of degradation temperature of compounds in a composting process and microbiological quality of the compost. Rev Bras Eng Agrícola e Ambient 17:54–59. https://doi.org/10.1590/S1415-43662013000100008
Jones P, Martin M (2003) A review of the literature on the occurrence and survival of pathogens of animals and humans in green compost. The Waste and Resources Action Programme
Karnchanawong S, Nissaikla S (2014) Effects of microbial inoculation on composting of household organic waste using passive aeration bin. Int J Recycl Org Waste Agric 3:113–119. https://doi.org/10.1007/s40093-014-0072-0
Lafond S, Paré T, Dinel H, Schnitzer M, Chambers JR, Jaouich A (2002) Composting duck excreta enriched wood shavings: C and N transformations and bacterial pathogen reductions. J Environ Sci Health Part B Pestic Food Contam Agric Wastes 37:173–186. https://doi.org/10.1081/PFC-120002989
Larney FJ, Yanke LJ, Miller JJ, McAllister TA (2003) Fate of coliform bacteria in composted beef cattle feedlot manure. J Environ Qual 32:1508–1515. https://doi.org/10.2134/jeq2003.1508
Mensah PY, Kuffour RA, Baidoo PK, Awuah E (2013) The effect of different percentages of bulking agent (sawdust) on microbial quality of faecal sludge. Water Sci Technol 67:1728–1733. https://doi.org/10.2166/wst.2013.047
Millner P, Ingram D, Mulbry W, Arikan OA (2014) Pathogen reduction in minimally managed composting of bovine manure. Waste Manag 34:1992–1999. https://doi.org/10.1016/j.wasman.2014.07.021
Neklyudov AD, Fedotov GN, Ivankin AN (2008) Intensification of composting processes by aerobic microorganisms: a review. Appl Biochem Microbiol 44:6–18. https://doi.org/10.1007/s10438-008-1002-6
Nennich TD, Harrison JH, VanWieringen LM, Meyer D, Heinrichs AJ, Weiss WP, St-Pierre NR, Kincaid RL, Davidson DL, Block E (2005) Prediction of manure and nutrient excretion from dairy cattle. J Dairy Sci 88:3721–3733. https://doi.org/10.3168/jds.S0022-0302(05)73058-7
Noble RT, Leecaster MK, McGee CD, Weisberg SB, Ritter K (2004) Comparison of bacterial indicator analysis methods in stormwater-affected coastal waters. Water Res 38:1183–1188. https://doi.org/10.1016/j.watres.2003.11.038
Ogunwande G, Osunade J, Adekalu KO, Ogunjimi L (2008) Nitrogen loss in chicken litter compost as affected by carbon to nitrogen ratio and turning frequency. Bioresour Technol 99:7495–7503. https://doi.org/10.1016/j.biortech.2008.02.020
Omrani MAAG, Samavat MSS (2017) Comparison between aerated static piles and vermicomposting in producing co-compost from rural organic wastes and cow manure. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-017-1607-5
Park GW, Diez-Gonzalez F (2003) Utilization of carbonate and ammonia-based treatments to eliminate Escherichia coli O157:H7 and Salmonella Typhimurium DT104 from cattle manure. J Appl Microbiol 94:675–685. https://doi.org/10.1046/j.1365-2672.2003.01899.x
Peters J, Combs S, Hoskins B, Jarman J, Kovar J, Watson M, Wolf A, Wolf N (2003) Recommended methods of manure analysis. A3769, p 62. https://soils.wisc.edu/extension/pubs/A3769.pdf
Raj D, Antil RS (2011) Evaluation of maturity and stability parameters of composts prepared from agro-industrial wastes. Bioresour Technol 102:2868–2873. https://doi.org/10.1016/j.biortech.2010.10.077
SAGARPA (2016) Población ganadera bovino carne y leche (Bovine meat and milk livestock inventory, Mexico). Servicio de Información Agroalimentaria y Pesquera (SIAP)
SEMARNAT (2002) Norma Oficial Mexicana NOM-004-SEMARNAT-2002, Protección ambiental. Lodos y biosólidos—Especificaciones y límites máximos permisibles de contaminantes para su aprovechamiento y disposición final. Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT)
Shepherd MW, Liang P, Jiang X, Doyle MP, Erickson MC (2010) Microbiological analysis of composts produced on South Carolina poultry farms. J Appl Microbiol 108:2067–2076. https://doi.org/10.1111/j.1365-2672.2009.04610.x
Truong THH, Marschner P (2018) Respiration, available N and microbial biomass N in soil amended with mixes of organic materials differing in C/N ratio and decomposition stage. Geoderma 319:167–174. https://doi.org/10.1016/j.geoderma.2018.01.012
USEPA (2001) Development document for the proposed revisions to the National Pollutant Discharge Elimination System Regulation and the Effluent Guidelines for Concentrated Animal Feeding Operations. 266. EPA-821-B-01-001
Vázquez MA, Soto M (2017) The efficiency of home composting programmes and compost quality. Waste Manag 64:39–50. https://doi.org/10.1016/j.wasman.2017.03.022
Venegas-González J, Cajuste JL, Trinidad-Santos A, Gavi-Reyes F, Sánchez-García P (2005) Chemical analysis of compost and the effect of its addition on biomass production in blackberry. TERRA Latinoam 23:285–291
Vochozka M, Maroušková A, Šuleř P (2017) Obsolete laws: economic and moral aspects, case study—composting standards. Sci Eng Ethics 23:1667–1672. https://doi.org/10.1007/s11948-016-9831-9
Wichuk KM, McCartney D (2007) A review of the effectiveness of current time–temperature regulations on pathogen inactivation during composting. J Environ Eng Sci 6:573–586. https://doi.org/10.1139/S07-011
Acknowledgements
Financial support was provided by the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP), Mexico (Grant No. 12294732544 Fondos Fiscales). Thank you to Tomas Rivas for sample collection and laboratory analyses and to the anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Macias-Corral, M.A., Cueto-Wong, J.A., Morán-Martínez, J. et al. Effect of different initial C/N ratio of cow manure and straw on microbial quality of compost. Int J Recycl Org Waste Agricult 8 (Suppl 1), 357–365 (2019). https://doi.org/10.1007/s40093-019-00308-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-019-00308-5