Abstract
Background
Water hyacinth (Eichhornia crassipes) is one of the worst weeds in the world. Composting is one of the most economical ways for the treatment and final disposal of water hyacinth, because it combines material recycling and biomass disposal. A major restriction of land application of water hyacinth compost is the possible high heavy metal concentration in the final product. Zeolites may be useful as metal scavengers in metal-rich water hyacinth compost. The speciation of heavy metals (Zn, Cu, Mn, Fe, Pb, Ni, Cd and Cr) was done according to Tessier’s sequential extraction method during water hyacinth composting mixed with cattle manure, sawdust and natural zeolite. The water hyacinth, cattle manure and sawdust were taken in the 6:3:1 ratio with 5, 10 and 15 % natural zeolite, respectively.
Results
The temperature was measured in the range of 48.6–56.2 °C in control and zeolite treatments. Higher reduction of exchangeable (F1) and oxidizable (F4) fractions of Zn was observed about 72.8 and 79.6 %, respectively, in zeolite 1 treatment but higher reduction of carbonate (F2) and reducible (F3) fractions was observed about 70 and 37 %, respectively, in zeolite 2 treatment. The F1, F2, F3 and F4 fractions of Cu and Fe were reduced in the control and all zeolite treatments. The F1, F2, F3 and F4 fractions of Mn were reduced significantly in the all zeolite treatments in comparison to control. The bioavailability factor (BF) of Zn, Cu, Mn, Fe, Ni, Cd and Cr was reduced significantly in zeolite treatments in comparison to control. There was no significant change observed with BF of Pb in zeolite treatments. The total concentration of Pb was higher than Zn, Cu, Mn, Ni and Cr but its BF was lowest among the all these metals.
Conclusion
Addition of optimum percentage of natural zeolite was successful for enhancing degradation of organic matter and reducing bioavailability of heavy metals during water hyacinth composting mixed with cattle manure and sawdust. The bioavailable fractions of heavy metals were effectively correlated with pH and TOC in control and zeolite 1 treatment as compared to zeolite 2 and 3 treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The composting is one of the most common methods for treatment of water hyacinth. But due to the presence of toxic contaminants such as heavy metals in the final compost, application of its compost to agricultural land is restricted (Singh and Kalamdhad 2012a, 2013a, b). Water hyacinth can accumulate heavy metals in their body (Malik 2007). Heavy metals uptake through plants and successive accumulation along the food chain is a probable hazard to animal and human health, if heavy metal-contaminated compost has to be applied to soil (Wong and Selvam 2006). Therefore, the mobility and bioavailability-related eco-toxicity to plants depend strongly on their specific chemical forms rather than the total concentration (Nair et al. 2008). Sequential extraction of heavy metals during the composting process is a useful technique for determining the chemical speciation (Walter et al. 2006). Assessment of the chemical speciation of heavy metals in compost assists to evaluate their bioavailability and suitability for land application (Wong and Selvam 2006).
The zeolites are naturally occurring hydrated aluminosilicate minerals that occur as three-dimensional frameworks of SiO4 and AlO4 tetrahedra (Villasenor et al. 2011). The aluminium ion is small enough to occupy the position in the centre of the tetrahedron of four oxygen atoms, while the isomorphous replacement of Si4+ by Al3+ produces a negative charge in the lattice. The exchangeable cations such as Na, K or Ca help in balancing net negative charge. These cations are exchangeable with certain cations in solutions such as lead, thorium, cadmium, zinc, manganese and ammonium ion (Sprynskyy et al. 2007; Villasenor et al. 2011). The framework of zeolite contains various channels and cavities where cations and water molecules are located. The channel structure of zeolites acts as a molecular sieve for selective cation exchange (Tabatabaei et al. 2012).
The natural zeolite has been used widely for improvement of the physical and chemical properties of the compost and immobilization of heavy metals during the sewage sludge composting (Zorpas et al. 2000; Sprynskyy et al. 2007; Villasenor et al. 2011). Zeolite could improve the composting process due to its ability to increase the porosity of the substrate (Zorpas et al. 2000). It has the ability to readily uptake almost all heavy metals that are bound to the exchangeable and the carbonate fractions (Singh and Kalamdhad 2012b). Therefore, the aim of the present study was to reduce most bioavailable fractions of Zn, Cu, Mn, Fe, Ni, Pb, Cd and Cr using natural zeolite during the composting of water hyacinth mixed with cattle manure and sawdust.
Materials and methods
Feedstock materials
Water hyacinth, cattle manure (cow dung) and sawdust were used for the preparation of different compost mixtures. Water hyacinth was collected from the Amingoan industrial area near Indian Institute of Technology Guwahati campus, India. Cattle manure was obtained from dairy farm near the campus. Sawdust was purchased from nearby saw mill. Natural zeolite (powder form) was purchased from G. M. chemicals Pvt. Ltd., Gujarat, India. Singh and Kalamdhad (2012a, 2013a, b) suggested that mixture of 90 kg water hyacinth, 45 kg cattle manure and 15 kg sawdust was best combination for reduction of bioavailability of heavy metals during water hyacinth composting, therefore, in this study same combination was used with 5, 10 and 15 % zeolite. Prior to composting, the maximum particle size of the waste mixture was restricted to 1 cm to provide better aeration and moisture control. The waste composition of different treatments is given as follows: control (water hyacinth 90 kg + cattle manure 45 kg + sawdust 15 kg), zeolite 1 (control + 5 % zeolite), zeolite 2 (control + 10 % zeolite) and zeolite 3 (control + 15 % zeolite). Initial characterizations of zeolite were found as follows: pH 8.85 ± 0.05, electrical conductivity (dS/m) 1.58 ± 0.03, moisture content (%) 3.75 ± 0.15, volatile solids (%) 11.73 ± 0.03, nutrients in mg/kg (Na 7,100 ± 100, K 1,850 ± 100, Ca 6,575 ± 0.08 and Mg 1,175 ± 125), and concentration of total heavy metals in mg/kg (Zn 6.61 ± 0.54, Cu 1.15 ± 0.05, Mn 4.60 ± 0.10, Fe 8.10 ± 0.26, Pb 0.72 ± 0.065 and Cr 0.08 ± 0.003).
Design of agitated pile composting
Four different waste combinations were prepared into trapezoidal piles (length 2,100 mm, base width 350 mm, top width 100 mm and height 250 mm, having length to base width (L/W) ratio of 6). Composting period for agitated pile composting was decided 30 days (Singh and Kalamdhad 2012a, 2013a, b, c). Agitated piles contained approximately 150 kg of different waste combinations and it was manually turned on 3, 6, 9, 12, 15, 18, 21, 24, 27 and 30th day. The samples from the piles were collected after turning at 0, 6, 12, 18, 24 and 30th day during the composting process.
Experimental analysis
A digital thermometer was used for temperature monitoring during the composting process. Each sample was analyzed for the following parameters: pH (1:10 w/v waste: water extract), moisture content and total organic carbon (TOC) (Kalamdhad et al. 2009). The atomic absorption spectrometer (AAS) (Varian Spectra 55B) was used for analysis of Zn, Cu, Mn, Fe, Ni, Pb, Cd and Cr concentrations after digestion of 0.2 g sample with 10 mL of H2SO4 and HClO4 (5:1) mixture in block digestion system (Pelican Equipments, Chennai, India) for 2 h at 300 °C.
Sequential extraction method developed by Tessier et al. (1979) for heavy metal speciation was followed; according to this method, the metals bound to five fractions: exchangeable (F1), carbonate (F2), reducible (F3), oxidizable (F4) and residual (F5) fraction. The detail methodology of sequential extraction was followed as Singh and Kalamdhad (2012a). The bioavailability factor (BF) was calculated as follows:
All the results reported are the means of three replicates. Repeated measures treated with analysis of variance (ANOVA) were made using STATISTICA software. The objective of the statistical analysis was to determine the any significant differences among the parameters analyzed for different proportions.
Results and discussion
Physico-chemical analysis
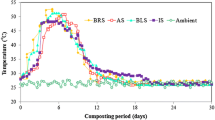
The variation in temperature profiles in control and all zeolite treatments during the composting process is shown in Fig. 1a. The temperature was measured in the range of 48.6–56.2 °C in control and zeolite treatments during the thermophilic phase due to the extreme microbial activity (Chiang et al. 2007). The thermophilic phase started slightly later in zeolite treatments in comparison to control but maximum thermophilic temperature of zeolite treatments was similar as control except zeolite 3. It could be explained as microbes take slightly more time for acclimatization with zeolite compost mixture than control. Higher addition of zeolite may absorb water, causing anaerobic condition for composting microorganisms (Villasenor et al. 2011); consequently, it reduced degradation of organic matter during the composting process.
Figure 1b shows the significant reduction in moisture content in control and all zeolite treatments during the process (F = 92.8, p < 0.05). The higher reduction of moisture content was observed in control (55.25 %) followed by zeolite 1 (52.38 %), zeolite 2 (50.66 %) and zeolite 3 (47.18 %) treatments. Lower reduction of moisture content in all zeolite treatments occurred in comparison to control due to moisture retained by zeolite during the process (Villasenor et al. 2011). Figure 1c illustrates the significant variation in pH values (F = 18.1, p < 0.001) from 6.38 to 7.85 during the composting process. The initial pH of compost mixture with zeolite was increased a little in comparison to control. The pH decreased slightly in the beginning of the composting process which may be due to acid formation during the decomposition of organic matter (Zorpas et al. 2000). The initial pH of zeolite feed mixtures was higher than control but at the end of composting process pH reduced and became neutral, similar as control. It might be due to the buffering capacity of composting process (Garg and Gupta 2011).
Figure 1d shows the significant reduction in TOC in control and all zeolite treatments during the process (F = 25.1, p < 0.001). The TOC was reduced from 43.51 to 33.50 %, 34.52 to 18.16 %, 30.17 to 17.04 % and 26.24 to 18.06 % in control, zeolite 1, 2 and 3 treatments, respectively. The higher reduction of TOC was found in zeolite 1 (47.4 %) and zeolite 2 (43.5 %) treatments during the composting process; it can be attributed as the biodegradation of organic matter was progressed by the addition of the zeolite (Villasenor et al. 2011). Zeolite has the ability to increase the porosity of the composting materials due to its bulking property, consequently enhanced the organic matter degradation (Zorpas et al. 2000). The TOC was lower in zeolite-treated compost as compared to the control, similar results also observed by Zorpas et al. (2000). TOC decreased during composting, which might be due to the microbial oxidation of the most labile forms of carbon, resulting in evolution of CO2 (Garcia et al. 1995).
Table 1 illustrates the variation in total concentration of metals (Zn, Cu, Mn, Fe, Ni, Pb, Cd and Cr) in control and all zeolite treatments during the composting process. The total concentration of metals was increased due to reduction of organic matter and release of CO2 during the mineralization processes (Zorpas et al. 2000). The variation in Zn, Cu, Mn, Fe, Ni, Pb, Cd and Cr concentrations in control and zeolite-amended compost was significant (F = 7.36, p < 0.001 for Zn, F = 42.72, p < 0.05 for Cu, F = 45.15, p < 0.05 for Mn, F = 50.59, p < 0.05 for Fe, F = 42.31, p < 0.05 for Ni, F = 8.96, p < 0.001 for Pb, F = 26.49, p < 0.001 for Cd, F = 4.37, p < 0.001 for Cr).
Speciation of heavy metals
According to Tessier et al. (1979) sequential extraction method, heavy metals are associated with five fractions: the F1 fraction which is likely to be affected by changes in ionic concentration as well as sorption–desorption processes; the F2 fraction which is most susceptible to changes in pH; the F3 fraction that is thermodynamically unstable under anoxic conditions; the F4 fraction that can be released under oxidizing conditions; the F5 fraction in which metals are permanently bound to crystal lattice of the mineral constituents of the compost. The F5 fraction will not be available for plants under the normal natural conditions.
Speciation of Zn, Cu, Mn, Fe and Ni
The Zn, Cu, Fe, Mn and Ni are essential elements required for normal growth and metabolism of plants, but high concentration of these elements may be toxic for plants (Singh and Kalamdhad 2011). Figures 2 and 3 show the speciation of Zn, Cu, Mn, Fe and Ni in control and zeolite 1 treatments, respectively. Tables 2 and 3 illustrate the speciation of these metals in zeolite 2 and zeolite 3 treatments, respectively, during the composting process.
The F1, F2 and F3 fractions of Zn were reduced in the control and zeolite 1 and 3 treatments. The F4 fraction was reduced in all treatments except zeolite 2 treatment during the process. It was increased in zeolite 2 treatment. Higher reduction of F1 (72.8 %) and F4 (79.6 %) fractions was observed in zeolite 1 treatment but higher reduction of F2 (70.0 %) and F3 (37.0 %) fractions was observed in zeolite 2 treatment. The F5 fraction of Zn was enhanced in all treatments. The order of different fractions of Zn in the final compost of control was: F5 > F4 > F3 > F2 > F1. In the zeolite 1 and 2 treatments the order was: F5 > F3 > F4 > F2 > F1, however, in zeolite 3 treatment the order was: F5 > F3 > F4 > F1 > F2. The F4 fraction of Zn was transformed into F3 and F5 fractions in all zeolite treatments. The BF of Zn was decreased from 0.75 to 0.48, 0.28 to 0.15, 0.21 to 0.13 and 0.20 to 0.15 in control and zeolite 1, 2 and 3 treatments, respectively (Fig. 4). The higher reduction of BF was observed in Zeolite 1 treatment (45.57 %) followed by zeolite 2 (38.41 %), control (36.37 %) and zeolite 3 treatments (27.30 %). Zorpas and Loizidou (2008) reported that Zn was mainly found in the F3 and F4 fractions in the sewage sludge compost with natural zeolite, however, in the present study it was mainly associated with the F5 fraction. The reduction of BF in zeolite 1 and 2 treatments might be due to the formation of Zn complex with various organic functional groups of humic substances formed during the maturity of compost (Singh and Kalamdhad 2012a). Kumpiene et al. (2008) suggested that cation exchange and complexation by organic ligands are the main Zn bioavailability-controlling mechanisms. The variation in F1, F2, F3, F4 and F5 fractions of Zn in control and zeolite treatments was significant (F = 20.7, p < 0.001 for F1, F = 15.0, p < 0.001 for F2, F = 12.3, p < 0.001 for F3, F = 45.6, p < 0.05 for F4, F = 5.04, p < 0.001 for F5).
The F1, F2, F3 and F4 fractions of Cu were reduced in the control and all zeolite treatments. Higher reduction of F1 (78.59 %) and F2 (83.54 % of total fraction) fractions were observed in zeolite 1 treatment. Higher reduction of F3 (77.88 %) and F4 (54.0 %) fractions were observed in zeolite 2 treatment. The F5 fraction of Cu was increased in all treatments during the process. The BF of Cu was decreased from 0.63 to 0.28, 0.15 to 0.06, 0.11 to 0.04 and 0.10 to 0.05 in control and zeolite 1, 2 and 3 treatments, respectively (Fig. 4). Higher reduction of BF was observed in zeolite 2 treatment (66.4 %) followed by zeolite 1 (63.0 %), control (55.5 %) and zeolite 3 treatments (52.6 %). This is caused by the uptake of easily available fractions of Cu by natural zeolite, while in zeolite 3 treatment results were not satisfactory. Zorpas et al. (2000) reported that a maximum amount of Cu was bound to the F4 and F5 fractions, while <2 % in the F1 and F2 fractions during sewage sludge composting with natural zeolite. While in the present study Cu was mostly bound to the F5 fraction. The F1 and F2 fractions contributed less that 2 % in all zeolite treatments, whereas in control these fractions were more than 2 %. Results reveal that addition of zeolite significantly reduced the F1 and F2 fractions of Cu during the composting process. The maximum reduction of movable fractions (from F1 to F4) of Cu was observed in zeolite 2 and 3 treatments; it happened by maximum organic matter loss resulting in conversion of organic matter into humic substances which have high contents of carboxyl groups (Liu et al. 2008). The variation in F1, F2, F3, F4 and F5 fractions of Cu in control and zeolite treatments was significant (F = 44.9, p < 0.001 for F1, F = 10.2, p < 0.001 for F2, F = 29.3, p < 0.001 for F3, F = 40.31, p < 0.001 for F4, F = 26.7, p < 0.001 for F5).
The F1, F2, F3 and F4 fractions of Mn were reduced in the all zeolite treatments in comparison with control. The F1 and F3 fractions were reduced while remaining mobile fractions (F2 and F4) were increased in the mature compost. The F5 fraction was increased in control and all zeolite treatments. The maximum reduction of F1 (70 %) and F2 (73.4 % of total fraction) fractions was observed in zeolite 2 treatment; whereas higher reduction of F3 and F4 fractions was observed in control and zeolite 3 treatments. The BF of Mn was decreased from 0.65 to 0.47, 0.84 to 0.60, 0.78 to 0.35 and 0.71 to 0.44 in control and zeolite 1, 2 and 3 treatments, respectively (Fig. 4). Higher reduction of BF was observed in zeolite 2 (55.0 %) followed by zeolite 3 (38.7 %), zeolite 1 (33.79) and control (26.5 %). Zorpas et al. (2000) reported approximately 60 % of Mn was bound to the F3 fraction during sewage sludge composting with natural zeolite despite the fact that in the present study the F5 fraction of Mn was dominant fraction and it was about 40–60 % of total fraction in control and zeolite treatments. It has been observed that the F5 fraction was increased in zeolite-added compost in comparison to control; it indicated that mobile fractions (F1 to F4) of Mn were transformed into most stable F5 fraction due to zeolite addition. The variation in the F1, F2, F3, F4 and F5 fractions of Mn in control and zeolite treatments was significant (F = 77.7, p < 0.05 for F1, F = 19.1, p < 0.001 for F2, F = 21.0, p < 0.001 for F3, F = 62.7, p < 0.05 for F4, F = 23.8, p < 0.001 for F5).
The F1, F2, F3 and F4 fractions of Fe were reduced in the control and all zeolite treatments. Higher reduction of F1 and F2 fractions was observed about 70.9 and 74.5 % of the total fraction in zeolite 2 and control treatments, respectively; but higher reduction of F3 and F4 fractions was observed about 58.4 and 68.4 % of the total fraction, respectively, in zeolite 1 treatment. The F5 fraction of Fe was increased in control and all zeolite treatments. The F1 fraction reduced significantly in all zeolite treatments compared to control.
The BF of Fe was decreased from 0.82 to 0.67, 0.41 to 0.14, 0.37 to 0.15 and 0.42 to 0.20 in the control and zeolite 1, 2 and 3 treatments, respectively (Fig. 4). Higher reduction of BF was observed in zeolite 1 treatment (63.4 %) followed by zeolite 2 (61.1 %), zeolite 3 (53.1 %) and control (18.8 %). It has been observed that higher BF was reduced in all zeolite treatments in comparison with control, which suggested that the addition of natural zeolite could check the mobility and bioavailability of Fe during the composting process. The F5 fraction of Fe was dominant (about 84–91 % of total fraction) in all zeolite-amended compost, however, in the control F4 fraction was dominant. Zorpas et al. (2000) reported that Fe was bound to the F3 and F5 fractions (about 95 % for the Fe). Zorpas and Loizidou (2008) reported that the order of Fe fraction was: F5 (64.19 %) > F3 (27.21 %) > F4 (7.95 %) > F2 (0.38 %) > F1 (0.27 %) in sewage sludge composting with natural zeolite. In the present study, this order was: F5 (86.0 %) > F4 (10.5 %) > F3 (3.5 %) > F1 (0.020 %) > F2 (0.017 %). The variation in F1, F2, F3, F4 and F5 fractions of Fe in control and zeolite treatments was significant (F = 20.6, p < 0.001 for F1, F = 19.4, p < 0.001 for F2, F = 30.6, p < 0.001 for F3, F = 42.4, p < 0.001 for F4, F = 11.7, p < 0.001 for F5).
The F1 and F2 fractions of Ni were reduced in the control and all zeolite treatments. Higher reduction of these fractions was observed about 64 and 67 % of the total fraction in zeolite 1 and 2 treatments, respectively; it could be explained as zeolite has taken up the Ni bound to the F1 and F2 fractions (Zorpas and Loizidou 2008). The F5 fraction of Ni was increased in control and all zeolite treatments. The F1 fractions contribute about 5 % of the total fraction in final compost of control, however, this fraction contributes <2 % of total fraction in zeolite treatments. The F2 fractions contribute about 1.5 % of the total fraction in the final compost of control, but this fraction contributes <1 % of the total fraction in zeolite treatments. Similar results were also reported by Zorpas et al. (2000) in the sewage sludge compost with natural zeolite.
The BF of Ni was decreased from 0.12 to 0.06, 0.06 to 0.02, 0.07 to 0.03 and 0.033 to 0.015 in control and zeolite 1, 2 and 3 treatments, respectively (Fig. 4). Highest reduction of BF was observed in zeolite 1 treatment (64.5 %) followed by zeolite 2 treatment (62.8 %), zeolite 3 treatment (54.2 %) and control (46.9 %). Higher reduction of BF in all zeolite treatments in comparison with control indicated that zeolite could inhibit the bioavailability of Ni during the composting process. Zorpas and Loizidou (2008) reported that Ni was found to be associated with the F3 fraction (36 %) and the F5 fraction (23 %) during the sewage sludge compost with natural zeolite. In the present study, Ni was mostly bound to the F5 fraction (about 93–98 % of total fraction), whereas F3 and F4 fractions were not detected throughout the composting process. The variation in F1, F2 and F5 fractions of Ni in control and zeolite treatments was significant (F = 18.6, p < 0.001 for F1, F = 19.0, p < 0.001 for F2, F = 20.1, p < 0.001 for F5).
Speciation of Pb, Cd and Cr
The Cr, Pb and Cd are not required for plant growth but even very less concentration of these metals can be toxic for plants and humans (Singh and Kalamdhad 2011). Figures 2 and 3 show the speciation of Pb, Cd and Cr in control and zeolite 1 treatments during the process, respectively. Tables 2 and 3 illustrate the speciation of these metals in zeolite 2 and 3 treatments, respectively, during the process.
The F1 and F2 fractions of Pb were reduced in the control and all zeolite treatments. Higher reduction of F1 (71.0 %) and F2 (67.8 % of total fraction) fractions was in zeolite 1 and 2 treatments, respectively, during the composting process; it might be due to these fractions taken up from the zeolite and transferred to the more stable fractions (Zorpas and Loizidou 2008). The F5 fraction of Pb was increased (percentage of total fraction) in all zeolite treatments and it was reduced in control. Zorpas et al. (2000) reported that Pb bound to the F3 and F5 fractions (83 % for the Pb); on the other hand, in the present study Pb was mainly bound to the F5 fraction (about 97–98 % of total fraction). The F3 and F4 fractions of Pb were not found during the composting process. The BF of Pb was decreased from 0.056 to 0.025, 0.044 to 0.013, 0.040 to 0.013 and 0.048 to 0.023 in control and zeolite 1, 2 and 3 treatments, respectively, during the process (Fig. 4). Higher reduction of BF was observed in zeolite 1 treatment (69.6 %) followed by zeolite 2 (67.2 %), control (51 %) and zeolite 3 (51.6 %) during the composting process. The reduction in BF of Pb in zeolite 1 and 2 treatments might be due to higher degradation of organic matter and conversion into humic substances resulting in the reduction of mobile fractions (F1, F2, F3 and F4) of Pb during the process. The lower values of BF were observed in all zeolite treatments when compared with control, confirmed that zeolite was efficient for reducing bioavailability of Pb in the mature compost. The variation in F1, F2 and F5 fractions of Pb in control and zeolite treatments was significant (F = 56.3, p < 0.05 for F1, F = 13.2, p < 0.001 for F2, F = 16.4, p < 0.001 for F5).
The F1 and F2 fractions of Cd were reduced in the control and all zeolite treatments during the process. Higher reduction of F1 and F2 fractions was observed about 72.2 and 69.8 % of total fraction in zeolite 1 and 2 treatments, respectively; it could be attributed to the ion-exchange reactions in the microporous minerals of the zeolite (Erdem et al. 2004). The F5 fraction of Cd was increased (percentage of total fraction) in all zeolite treatments although it was reduced in control during the composting process. According to Zorpas et al. (2000) about 60 % of Cd was bound to the F3 fraction during sewage sludge composting with natural zeolite, while in the present study the F5 fraction of Cd was dominant and contributed about 98 % of the total fraction. The F1 and F2 fractions of Cd was contributed about 3.4 and 1.9 % of total fraction, respectively, in the final compost of control; however, these fractions were assessed <1 % of total fraction each in the final compost of zeolite treatments. The reduction in most bioavailable fractions of Cd attributed as enhancement of initial pH in all zeolite treatments.
The BF of Cd was decreased from 0.06 to 0.053, 0.035 to 0.014, 0.033 to 0.014 and 0.033 to 0.015 in control and zeolite 1, 2 and 3 treatments, respectively, during the process (Fig. 4). Higher reduction of BF was observed in zeolite 1 treatment (61.3 %) followed by zeolite 2 (59.1 %), zeolite 3 (53.9 %) and control (10.5 %). Higher reduction of BF was observed in all zeolite treatments as compared to control it may be attributed as zeolite taken up easily available fractions of Cd; furthermore, higher degradation of organic matter and conversion into humic substances result in binding of mobile fractions (F1, F2, F3 and F4) of Cd with zeolite. The variation in F1, F2 and F5 fractions of Cd in control and zeolite treatments was significant (F = 50.3, p < 0.05 for F1, F = 13.0, p < 0.001 for F2, F = 3.1, p = 0.002 for F5).
The F1 and F2 fractions of Cr were reduced in the control and zeolite treatments. The F3 and F4 fractions were reduced in control and zeolite 1 treatments, while these fractions were increased in zeolite 2 and 3 treatments during the composting process. Higher reduction of F1 and F3 fractions was observed about 60.7 and 69.5 % of the total fraction in zeolite 2 treatment, and the maximum reduction of F2 fraction was observed about 76.1 % of total fraction in zeolite 1 treatment. The F5 fraction of Cr was increased in control and all zeolite treatments. The BF of Cr was decreased from 0.29 to 0.18, 0.15 to 0.06, 0.11 to 0.06 and 0.11 to 0.07 in control and zeolite 1, 2 and 3 treatments, respectively, during the process (Fig. 4). Higher reduction of BF was observed in zeolite 1 treatment (62.7 %) followed by zeolite 2 (46.9 %), zeolite 3 (38.6 %) and control (36.4 %) during the composting process. The higher reduction of BF was observed in all zeolite treatments in comparison with control in the final compost. Zorpas et al. (2000) reported that a maximum amount of Cr was bound to the F4 and F5 fractions while <2 % in the F1 and F2 fractions in the zeolite-amended sewage sludge compost. Despite the fact that in the present study Cr was mainly associated with F5 fraction. The F1 and F2 fractions of Cr was observed about 9.1 and 4.0 % of total fraction, respectively, in the final compost of control; whereas these fractions were measured <2.5 and 1.5 % of total fraction, respectively, in the final compost of zeolite treatments. The higher reduction in BF of Cr in zeolite 1 and 2 treatments might be due to the higher degradation of organic matter and reduction in readily available fractions (F1 and F2) of Cr during process. Furthermore, it could be attributed as zeolite has the ability to absorb and exchange Cr with other cations (Na, K and Ca) (Zorpas and Loizidou 2008). The variation in F1, F2, F3, F4 and F5 fractions of Cr in control and zeolite treatments was significant (F = 8.0, p < 0.001 for F1, F = 48.8, p < 0.001 for F2, F = 16.1, p < 0.001 for F3, F = 17.3, p < 0.001 for F4, F = 19.2, p < 0.001 for F5).
Correlation analysis between pH, TOC and bioavailable fractions of heavy metals
The bioavailabilities of heavy metals depend on pH and TOC during the composting process, so the linear correlation was analyzed between pH, TOC and bioavailable fractions (F1, F2, F3 and F4). Figure 5 illustrates linear correlation of pH with different fractions of Zn and Cu in control and zeolite treatments. The F1 and F2 fractions of Zn were correlated with pH in zeolite 1 treatment (88.5 % for F1 and 87.9 % for F2). Very poor correlation was observed in zeolite 2 and 3 treatments. An inverse relation was found between all fractions (except F3) and pH in zeolite 1 treatment. All fractions of Cu were negatively correlated with pH in zeolite 1 treatment. The F3 and F1 fractions of Cu were well correlated with pH in zeolite 1 and 3 treatments, respectively (83.9 % for F1 and 87 % for F3), however, all fractions of Cu were poorly correlated (<0.5 %) with pH in zeolite 2 treatment. There was no strong correlation observed between all fractions of Mn and pH in control and all treatments. The F2 fraction of Mn was not correlated with pH in zeolite 2 treatment (Fig. 6). The F1 fraction of Fe was not correlated with pH in zeolite 2 and 3 treatments, while it was correlated (>70 %) with pH in control and zeolite 1 treatments (Fig. 6). All fractions of Fe were correlated with pH in zeolite 1 treatment (75.8 % for F1, 84.8 % for F2, 77.1 % for F3 and 72.7 % for F4).
Figure 7 illustrates linear correlation of pH with F1 and F2 fractions of Ni and Pb in control and zeolite treatments. The correlation of pH with F3 and F4 fractions of Ni, Pb and Cd was not applicable. The F1 and F2 fractions of Ni weakly or not correlated with pH in zeolite 2 and 3 treatments, on other hand these fractions were correlated with pH in control and zeolite 1 treatment. The F1 and F2 fractions of Pb were negatively correlated with pH in zeolite 1 treatment (84.2 % for F1 and 79.8 % for F2). The F1 fraction of Pb does not depend on pH in the zeolite 2 treatment. The F1 and F2 fractions of Cd were correlated strongly in control in comparison with zeolite treatments (Fig. 8), but dependency of these fractions with pH was very less (<1 %) as found in zeolite 2 treatment. Similar to other metals, different fractions of Cr were not effectively correlated with pH in zeolite 2 treatment (Fig. 8). A negative correlation of F1 and F2 fractions of Cr was examined in zeolite 2 treatment (82.6 % for F1 and 64.2 % for F2).
Table 4 illustrates linear correlation of TOC with different fractions of Zn, Cu, Mn, Fe, Ni, Pb, Cd and Cr in control and zeolite treatments. The TOC was strongly correlated with F1 and F2 fractions of Zn in zeolite 1 treatment (95.6 % for F1 and 97.4 % for F2). Very poor correlation of different fractions of Zn was found in control and zeolite 3 treatments. Strong correlation of TOC with F3 fraction of Cu was observed in zeolite 1 treatment (96.8 % for F3). The F1 and F2 fractions of Mn were strongly correlated with TOC in zeolite 3 treatment (94.9 % for F1 and 97.7 % for F2). Poor correlation of different fractions of Mn with TOC was found in control as compared with zeolite treatments. F1 fraction of Mn was correlated with TOC zeolite 3 treatment (96.4 % for F1). The F2 fraction of Fe was correlated with TOC 90.0, 91.5 and 95.9 % in zeolite 1, 2 and 3 treatments, respectively. The correlation of TOC with F3 and F4 fractions of Ni, Pb and Cd was not applicable. The F1 and F2 fractions of Ni were correlated with TOC in the range of 73.2–92.9 % in zeolite 1 and zeolite 2 treatments. The F1 fraction of Pb was correlated with TOC about 91.8 and 92.2 % in zeolite 1 and 2 treatments, respectively, while F2 fraction was correlated with TOC about 93.5 and 92.6 % in control and zeolite 1 treatments. The F1 and F2 fractions of Cd were correlated with TOC about 99.9 and 89.4 % in zeolite 3 treatment, whereas very poor correlation was found in the control. The F1, F2, F3 and F4 fractions of Cr were correlated with TOC significantly in zeolite 3 treatment (about 85–95 %) in comparison with control, zeolite 1 and 2 treatment.
Conclusion
Natural zeolite addition enhanced the initial pH and organic matter degradation, and the resulting speciation of heavy metals was affected during the composting process. The bioavailability factor of all selected heavy metals was reduced significantly in zeolite 1 and 2 treatments in comparison with control and zeolite 3 treatments. The order of BF for different metals in the compost of control was: Fe (0.66) > Zn (0.55) > Mn (0.47) > Cu (0.28) > Cr (0.18) > Ni (0.06) > Cd (0.053) > Pb (0.025), however, order of these metals in zeolite treatments was: Mn (0.35) > Fe (0.19) > Zn (0.15) > Cr (0.066) > Cu (0.060) > Ni (0.026) > Cd (0.023) = Pb (0.023). The total concentration of Pb was higher than Zn, Cu, Mn, Ni and Cr, but its BF was lowest among the all these metals. The total concentration of Cu and Cr was much lower than Pb and Ni but their BF were higher than Pb and Ni in the mature compost. The total concentration of Pb was eighteen times of Cd but BF of both metals was found to be equal. The order of BF in the control and zeolite treatments indicates that BF of heavy metals reduced as a result of zeolite addition and also exposes that toxicity of metals depends on its bioavailable fractions rather than total metal concentration. Therefore, this study concluded that the use of natural zeolite was successful for reducing bioavailability of heavy metals during water hyacinth composting mixed with cattle manure and sawdust. The conclusion from correlation analysis shows that bioavailable fractions of heavy metals were dependent effectively on pH and TOC in control and zeolite 1 treatments in comparison with zeolite 2 and 3 treatments. Unsatisfactory correlation of pH with different fractions was observed in zeolite 2 treatment.
Authors’ contributions
First author Jiwan Singh completed experimental work and co-author Dr. Ajay S. Kalamdhad guided during this research.
References
Chiang KY, Huang HJ, Chang CN (2007) Enhancement of heavy metal stabilization by different amendments during sewage sludge composting process. J Environ Eng Manage 17(4):249–256
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280:309–314
Garcia C, Moreno JL, Hernfindez T, Costa F (1995) Effect of composting on sewage sludges contaminated with heavy metals. Bioresour Technol 53:13–19
Garg VK, Gupta R (2011) Optimization of cow dung spiked pre-consumer processing vegetable waste for vermicomposting using Eisenia fetida. Ecotoxicol Environ Saf 74:19–24
Kalamdhad AS, Singh YK, Ali M, Khwairakpam M, Kazmi AA (2009) Rotary drum composting of vegetable waste and tree leaves. Bioresour Technol 100:6442–6450
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As Cr Cu Pb and Zn in soil using amendments—a review. Waste Manage 28:215–225
Liu S, Wang X, Lu L, Diao S, Zhang J (2008) Competitive complexation of copper and zinc by sequentially extracted humic substances from manure compost. Agric Sci China 7(10):1253–1259
Malik A (2007) Environmental challenge vis a vis opportunity: the case of water hyacinth. Environ Int 33:122–138
Nair A, Juwarkar AA, Devotta S (2008) Study of speciation of metals in an industrial sludge and evaluation of metal chelators for their removal. J Hazard Mater 152:545–553
Singh J, Kalamdhad AS (2011) Effects of heavy metals on soil plants human health and aquatic life. Int J Res Chem Environ 1(2):15–21
Singh J, Kalamdhad AS (2012a) Concentration and speciation of heavy metals during water hyacinth composting. Bioresour Technol 124:169–179
Singh J, Kalamdhad AS (2012b) Reduction of heavy metals during composting—a review. Int J Environ Prot 2(9):36–43
Singh J, Kalamdhad AS (2013a) Assessment of bioavailability and leachability of heavy metals during rotary drum composting of green waste (Water hyacinth). Ecol Eng 52:59–69
Singh J, Kalamdhad AS (2013b) Bioavailability and leachability of heavy metals during water hyacinth composting. Chem Speciat Bioavail 25(1):1–14
Singh J, Kalamdhad AS (2013c) Effects of lime on bioavailability and leachability of heavy metals during agitated pile composting of water hyacinth. Bioresour Technol 138:148–155
Sprynskyy M, Kosobucki P, Kowalkowski T, Buszewsk B (2007) Influence of clinoptilolite rock on chemical speciation of selected heavy metals in sewage sludge. J Hazard Mater 149:310–316
Tabatabaei SH, Najafi P, Mirzaei SMJ, Nazem Z, Heidarpour M, Hajrasoliha S, Afyuni M, Harchegani HB, Akasheh LE, Zamanian M, Barani M, Amini H (2012) Compost’ leachate recycling through land treatment and application of natural Zeolite. Int J Recycl Org Waste Agric 1(2):1–7
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedures for the speciation of particulate trace metals. Anal Chem 51:844–851
Villasenor J, Rodriguez L, Fernandez FJ (2011) Composting domestic sewage sludge with natural zeolites in a rotary drum reactor. Bioresour Technol 102(2):1447–1454
Walter I, Martinez F, Cala V (2006) Heavy metal speciation and phytotoxic effects of three representative sewage sludge for agricultural uses. Environ Pollut 139:507–514
Wong JWC, Selvam A (2006) Speciation of heavy metals during co-composting of sewage sludge with lime. Chemosphere 63:980–986
Zorpas AA, Constantinides T, Vlyssides AG, Haralambous I, Loizidou M (2000) Heavy metal uptake by natural zeolite and metals partitioning in sewage sludge compost. Bioresour Technol 72:113–119
Zorpas AA, Loizidou M (2008) Sawdust and natural zeolite as a bulking agent for improving quality of a composting product from anaerobically stabilized sewage sludge. Bioresour Technol 99:7545–7552
Acknowledgments
The authors gratefully acknowledge the financial support of the Department of Science and Technology (DST), Government of India.
Conflict of interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Singh, J., Kalamdhad, A.S. Effects of natural zeolite on speciation of heavy metals during agitated pile composting of water hyacinth. Int J Recycl Org Waste Agricult 3, 55 (2014). https://doi.org/10.1007/s40093-014-0055-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40093-014-0055-1