Abstract
In recent years, extensive work is being conducted to improve the biodegradability index (BI) of effluent to enhance the efficiency of biochemical treatment. Advanced oxidation processes are one among the various methods used as primer to improve the BI of organic effluent. In the present investigation, experiments such as electrocoagulation, electro-oxidation and photochemical were carried out to treat pulp and paper wastewater in a wide range of operating conditions. The effect of individual parameters on BI improvement was critically examined. It was noticed that electro-oxidation method yields maximum biodegradability improvement (0.13–0.42) within process time of 35 min.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulp and paper industry is a highly capital, energy, and water intensive industry, also a highly polluting process and requires substantial investments in pollution control equipments. Looking into the serious nature of pollution, the pulp and paper industry in India has been brought under the 17 categories of highly polluting industries. India produces 6 million tonnes of paper per year though 311 mills by consuming around 900 million m3 of water and discharging 700 million m3 of wastewater. Out of these about 270 small paper mills (capacity ≤10,000 tonnes per annum (TPA), having a total installed capacity of 1.47 MTPA) do not have chemical recovery units [1]. Effluents from this industry cause alternations in hydrographical parameters of the water body thereby causing tremendous to the ecosystem. The sources of pollution among various process stages in pulp and paper industry are wood preparation, pulping, pulp washing, bleaching, and paper machine and coating operations. Common pollutants include suspended solids, oxygen demanding wastes, colour, basicity, heavy metals, alkali and alkaline earth metals, phenols, chloro-organics, cyanide, sulphides and other soluble substances [2]. Recent progress in the treatment of persistent organic pollutants in wastewater has led to the development of advanced oxidation processes (AOPs). Advanced oxidation processes make use of strong oxidants to reduce COD/BOD levels, and to remove both organic and oxidizable inorganic components. The processes can completely oxidize organic materials to carbon dioxide and water with the help of free hydroxyl radicals (OH· + OH−). Advanced oxidation process offer several advantages like process operability, absence of secondary waste and soil remediation. This method of treatment can be used either as a main treatment or as a hybrid technique [3]. It can also be used as a pre-treatment scheme for difficult wastewater for which feasible treatment methods are not available.
There are various methods available for treatment which includes biological method; physical methods like adsorption, membrane filtration; and chemical oxidation methods like electro-oxidation [4]. In advanced oxidation technique formation of strong oxidants plays an important role for the breakdown of pollutants into simple compounds [5]. Hydroxyl radicals can be produced by various methods such as electro-oxidation, photochemical and ozonation. Effectiveness of techniques is proportional to the ability to generate hydroxyl radicals.
In this present investigation, three different methods, i.e. electrocoagulation, electro-oxidation and photochemical methods were carried out to treat pulp and paper effluent. Electrocoagulation process is the in situ production of coagulants by means of electrolysis. Electro-oxidation is a process of mineralizing pollutants by electrolysis using anodes. It is of two types: direct oxidation and indirect oxidation. In direct method, anodic electron transfer takes place similar to chemical oxidation on anodic surface. In indirect method demineralization takes place in the presence of ferric and chloride ions [6]. Photolysis involves the interaction of light with molecules to bring about their dissociation into fragments. The addition of energy as radiation to a chemical compound is the principle of photochemical processes. Molecules absorb this energy and reach excited states with decay times long enough to take part in chemical reactions.
In this present investigation, improvement of biodegradability index using electrocoagulation and different advanced oxidation processes was studied. The influence of individual parameters on treatment was analysed. Significance of the method was also analysed and reported.
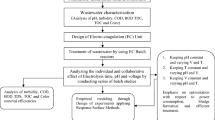
Materials and methods
The pulp and paper wastewater was used for the present investigation. The characteristics of the wastewater are given in the Table 1. Reactor consists of electrodes of 4.5 × 5.5 cm in size where mild steel was used as anode for electrocoagulation and graphite for electro-oxidation. Stainless steel was used as cathode for both the processes. Experiments were carried out in a batch electrochemical reactor of 250 ml capacity. The active surface area of the electrode was 25 cm2 and anode–cathode distance was maintained at 1 cm. 1 g l−1 of sodium chloride was used as supporting electrolyte. For mixing the reactor contents, a 1.5-cm-long stirring bead was used and the reactor was placed over a magnetic stirrer. The DC power supply system used was capable of supplying DC power in the range of 0–32 V/0–10 A.
Photochemical reactor setup consists of UV irradiation source as an 8-Watt lamp and its maximum emission is at 365 nm. Experiments were carried out in a batch photochemical reactor. Effluent has been taken in sample tube of capacity 0.1 L. H2O2 was used as an oxidant, which was added externally to enhance the efficiency of the photochemical process. The samples were irradiated for a period of 3 h (180 min) with a sampling interval of 30 min. The sample was immediately analysed for percentage COD removal.
Results and discussion
The wastewater from pulp and paper industry was collected and treated using three different techniques namely electrocoagulation, electro-oxidation, photochemical methods. Each treatment techniques were optimized with respect to various operating conditions. Comparisons between techniques have been done depending on improvement of biodegradability index with process time.
Electrocoagulation
Electrocoagulation is a synergistic process with a complex mechanism operating to remove pollutants from the water. Electrocoagulation operates by the dissolution of metal from the anode and with simultaneous formation of hydroxyl ions and evolution of hydrogen gas at the cathode. The hydroxide flocculates and coagulates the suspended solids thereby purifying the water. The generated ferric ions form monomeric, ferric hydroxo complexes with hydroxide ions and polymeric species, depending on the pH range. The Fe(OH)3 flocs capture the pollutant molecule present in the wastewater to form sludge as shown in the following reaction:
To optimize the electrocoagulation operating parameters, the reduction of COD with electrolysis time has been analysed. Experiments were carried out at different pH, current densities and supporting electrolyte. Figure 1 shows the influence of pH on %COD removal. Reduction in COD percentage was high at neutral pH. At higher value of pH, COD reduction was not significant. This is due to the reason that at higher pH value, the solubility of complex formed increases which do not involve in the COD removal [7].
Figure 2 depicts the influence of various current densities on COD reduction. It has been noted that COD removal increases as current density increases from 5 to 10 mA cm−2. It can be noticed that increasing the current density beyond 10 mA cm−2 did not show any significant improvement in the percentage COD removal because of unwanted side reactions as the pollutant concentration decreases.
Figure 3 shows the BI analysis of electro coagulation using mild steel as an anode at neutral condition, and current density 10 mA cm−2. BI value improved from 0.13 to 0.4 within the process time of 40 min, needed for efficient bio degradation [8]. In electrocoagulation, there is an enhanced action on the COD removal by coagulation and adsorption of the pollutants by the dissolved anode, which in turn enhances the BOD to COD ratio, i.e., biodegradability index.
Electro-oxidation
The complex mechanism of electrochemical oxidation of wastewater involves the coupling of electron transfer reaction with a dissociate chemisorption step. Basically, two types of oxidative mechanism may occur at the anode; oxidation occurs at the electrode surface in the case of an anode with high electrocatalytic activity, called direct electrolysis; in the other case, oxidation occurs via the surface mediator generated continuously on the anodic surface, called indirect electrolysis (metal oxide electrode). The preferable way for wastewater treatment is the physisorbed route of oxidation. The organic hydrogen peroxides formed are relatively unstable and decomposes which lead to molecular breakdown and the formation of subsequent intermediates with lower carbon numbers [9]. This leads to an improvement in the biodegradability index of the wastewater and can be subjected to biological treatment efficiently.
For electro-oxidation method, three parameters such as current density, pH and supporting electrolyte were taken into consideration to determine their effects in COD reduction. Figure 4 explains the influence of pH on COD removal. It has been noticed that treatment at acidic condition gives maximum COD removal up to 86.5 % compared to neutral and basic conditions. Lower pH facilitates the formation of hydroxyl radicals and the organic material in the wastewater can be easily oxidized [10].
From the Fig. 5, it was clear that COD removal increases as current density increases from 5 to 10 mA cm−2. It has been noticed that increasing the current density beyond 10 mA cm−2 did not show any significant improvement in the percentage COD removal. At higher current densities, the efficiency of COD removal decreases after certain period of time. This is due to the fact that at higher current density, temperature increases and also organic pollutant concentration is lower which leads to side reactions.
From the Fig. 6, it can be understood that efficiency of COD removal increases with increase in supporting electrolyte concentration from 5 to 10 g l−1. At higher concentrations, intermediates were formed which do not contribute to COD removal. Also, as pollutant concentration decreases Cl− radicals in the effluent combine with remaining organic compounds and form complexes, this increases COD of the wastewater [11].
Figure 7 BI analysis has been made for optimal condition. BI has been improved from 0.13 to 0.4 within the process time of 35 min. After certain period of time, the proportion of degrading organic pollutants is decreased by oxidizing agents, resulting in increased value of BI. The larger molecules in the wastewater are broken down into smaller molecules by the oxidative mechanism and hence facilitate the better action of microorganism on to the organic molecules by biodegradation.
Photochemical reaction
In photochemical reaction, H2O2 and ferrous sulphate were added together for oxidation. Advance oxidation process (AOPs) are characterized by the efficient production of hydroxide radicals. The simplified primary reactions of photochemical H2O2/Fe2+-based oxidative water treatment process is given below:
The above reaction sequence shows the production of OH· radical which acts as an oxidant for the oxidative degradation of pollutants in the wastewater. The various operating parameters like pH and the concentration of H2O2 and Fe2+ has been studied. The effect of pH is given in Fig. 8. It has been noticed that treatment at acidic condition gives maximum COD removal up to 61 % compared to neutral and basic conditions. However, at pH greater than 4, the percentage COD removal decreased with increasing pH from 4 to 9. The hydrogen peroxide starts decomposing at higher pH values [12].
The effect of H2O2 concentration was studied by changing H2O2 concentration from 111 to 222 mg l−1. It has been noticed from the Fig. 9 that increase in the concentration of H2O2 increases the percentage COD removal from 111 to 150 mg l−1. After that it does not show significant increase in percentage COD removal. This is due to the fact that at higher concentration of H2O2, it destroys hydroxyl radicals formed [13].
From the Fig. 10, it can be noticed that percentage COD removal was increased for Fe2+ concentration from 25 to 75 mg l−1. At the higher concentrations of ferrous sulphate, Fe2+ combines with hydroxyl radicals which lead to side reactions [12]. From the above figures, it could be concluded that maximum percentage COD removal can be obtained at acidic pH 4 with 166.5 mg l−1 H2O2 and 50 mg l−1 Fe2+ concentrations.
BI analyses were made for treated effluent and are given in the Fig. 11. From the above figure, it can be seen that the BI Index reached the value of 0.4 only after the treatment time of 150 min, which shows that it is not as efficient as electrochemical methods in increasing the biodegradability index of the wastewater and is time consuming.
Conclusion
Though both the electrochemical treatment methods reach the BI of 0.4 at a treatment time of 35–40 min, the electro-oxidation method gives better improvement of BI within the processed time of 35 min without any sludge formation. Then, photochemical method also has disadvantages like usage of expensive UV lamp which is harmful to humans and also it is a very slow process. It can be concluded that the electro-oxidation method is a better option for the improvement of biodegradability index. The treated wastewater now can be further processed by biochemical technique.
References
Mahesh S, Prasad B, Mall ID, Mishra IM (2006) Electrochemical degradation of pulp and paper mill wastewater, COD and color removal. Ind Eng Chem Res 45:2830–2839
Achoka JD (2002) The efficiency of oxidation ponds at the Kraft pulp and paper mill at Webuye in Kenya. Water Res 36:1203–1212
Klavarioti M, Mantzavinos D, Kassinos D (2009) Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Int J 35:402–417
Parama Kalyani KS, Balasubramanian N, Srinivasakannan C (2009) Decolorization and COD reduction of paper industrial effluent using electrocoagulation. Chem Eng J 151:97–104
Bidhan C, Bag C, Sai M, Sekhar K, Bhattacharya C (2009) Treatment of wastewater containing pyridine released from N, N-Dichlorobis (2,4,6-trichlorophenyl) urea (CC2) plant by advanced oxidation. J Environ Prot Sci 3:34–40
Chithra K, Balasubramanian N (2010) Modeling of electrocoagulation through adsorption kinetics. J Model Simul Syst 1:124–130
Kobya M, Can OT, Bayramoglu M (2003) Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J Hazard Mater 100:163–178
Chamarro E, MarcoA Esplugas S (2001) Use of fenton reagent to improve organic chemical bio-degradability. Water Res 35:1047–1051
Yan L, Ma H, Wang B, Wang Y, Chen Y (2011) Electrochemical treatment of petroleum wastewater with three-dimensional multi-phase electrode. Desalination 276:397–402
Ahmed Basha C, Soloman PA, Velan M, Miranda Lima Rose, Balasubramanian N, Siva R (2010) Electrochemical degradation of specialty chemical industry effluent. J Hazard Mater 176:154–164
Miled W, Hajsaid A, Roudesli S (2010) Decolorization of high polluted textile wastewater by indirect electrochemical oxidation process. J Text Appar Technol Manag 6:89–97
Narendra Kumar B, Anjaneyulu Y, Himabindu V (2011) Comparative studies of degradation of dye intermediate (H-acid) using TiO2/UV/H2O2 and Photo-Fenton process. J Chem Pharma Res 3:718–731
Dincer AR, Karakaya N, Gunes N, Gunes Y (2008) Removal of cod from oil recovery industry wastewater by the advanced oxidation processes (aop) based on H2O2. J Glob NEST 10:31–38
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Asha, A., Keerthi, Muthukrishnaraj, A. et al. Improvement of biodegradability index through electrocoagulation and advanced oxidation process. Int J Ind Chem 5, 4 (2014). https://doi.org/10.1007/s40090-014-0004-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40090-014-0004-x