Abstract
In this paper, nickel oxide (NiO) nanoparticles have been fabricated using wet method and deposited on the surface of multi-walled carbon nanotube (MWCNT). To do so, functional groups were introduced on the surface of MWCNTs by treating with concentrated nitric acid. Nickel oxide nanoparticles were formed on the surface of functionalized MWCNTs by incipient wetness impregnation of nickel nitrate, and the resultant product was calcinated in air atmosphere. Characteristics of the NiO/MWCNT were examined by various techniques, for example, Fourier transform spectroscopy (FTIR), X-ray diffraction analysis (XRD), transmission electron microscopy (TEM), thermogravimetric analyzer (TGA), and nitrogen adsorption–desorption isothermal as well as vibrating sample magnetometer (VSM). The FTIR spectra showed that carboxyl and hydroxyl functional groups existed on the surface of MWNTs after modification by concentrated nitric acid. The pattern of XRD indicated that MWNTs and nickel oxide nanoparticles coexisted in the NiO/MWCNT sample. The TEM images revealed that the NiO nanoparticles were distributed on the surface of the MWNTs, with the size ranging from 5 to 60 nm. Thermogravimetric analysis proved that NiO content decorated on MWCNTs was 80 and 15 wt%. The results of the Brunauer–Emmett–Teller (BET) data showed that the slight increment in the specific surface areas and porosities in the presence of the NiO nanoparticles on the surface of CNT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon nanotubes (CNTs) have attracted a lot of attention since 1991 due to their unique molecular geometry, the excellent electronic, thermal, and mechanical properties [2, 3, 6, 13, 22, 24, 28]. Over the past two decades, their outstanding properties and significant potential applications in nanoscience and nanotechnology cause to carbon nanotubes (CNTs) have received a considerable attention in scientific studies and industrial applications [3, 6, 22, 28].
To improve the CNT properties, nanotubes have been treated in different ways, such as functionalizing, coating, and filling the pristine nanotubes [1, 5, 15]. Because of their interesting dimensions and high surface areas, various inorganic nanoparticles, such as metals, metal oxides, and semiconducting nanoparticles, were coated on the surface of CNTs. Decoration of the surface of CNTs can get better the dispersity of CNTs in solvents [18] as well as promotion their optical, electric, and magnetic properties [7, 19, 25, 26].
To develop high performance of CNT/metal oxide composites and increase their dispersion stability, surface modification of CNT is the first important step. The pure CNT has chemical stability and highly hydrophobic nature. Acid treatment as a chemical functionalization method causes to create carboxylic acid and hydroxyl groups on the surface of CNT, and it is concluded that there is good physical and electrical contact between the CNTs and metal oxide nanoparticles [1, 15].
To date, many researchers have attempted to produce metal or metal oxide nanoparticles attaching on the external surface of CNTs or filling inside the CNTs [4, 8, 9, 26, 27]. Nickel oxide (NiO), as an important transition metal oxide, is a very promising material and has attracted increasing attention due to its extensive important applications as catalysis [20], electrode materials [21], gas sensor [10, 17], and electrochemical films [11]. For example, Gao et al. presented that the electrochemical properties of NiO/CNTs composite could be improved greatly. They showed that the good electrochemical characteristics of NiO/CNTs composite and excellent cycle life can be attributed to three-dimensionally interconnected nanotubular structure with a thin film of electroactive materials [16].
This study presents the characteristics of NiO nanoparticles deposited on the surface of Multi-walled carbon nanotube (MWCNTs). For this purpose, CNTs were functionalized by acid treatment. Then, the opened CNTs were decorated using NiO nanoparticles through the wet chemistry method.
Experimental
Materials
MWCNT with specific diameters of 40–60 nm, specific length of 5–15 µm, and purity greater than 95 % was obtained from Nanolin, China. Ni(NO3)2·6H2O was purchased from Merck.
Procedure of acid treatment of MWNTs
0.1 grams of MWNTs were dispersed in 50 ml concentrated HNO3 (68 wt%). The mixture was sonicated in a water bath for 2 h at ambient temperature, and then was stirred at the same temperature for 2 h. Afterward, the mixture was filtered and washed by deionized water for several times until pH adjusted to 6. Opened MWNTs were dried at 90 °C for overnight.
The preparation of NiO/MWNT
Some oxidized MWNTs were added to a solution of Ni(NO3)2·6H2O in a water/methanol 4/1 v/v. The mixture was sonicated in a water bath at room temperature for 2 h, and then, it was settled at room temperature for 2 days. After impregnation and filtration, the mixture was dried at 50 °C for 10 h. The calcination of sample was performed at 350 °C for 2 h. The nickel oxide loadings were controlled by changing the concentration of the nickel nitrate in the solution.
Instruments
TEM observation with line resolution of 3 Å and operated at 120 kV was carried out on a TEM LEO 912 AB. The Fourier transform infrared spectroscopy measurement (FTIR) was performed on a magna-IR 560 E.S.P FT-IR spectrometer. The thermogravimetric analysis was studied using a thermal analysis system (TGA50, Shimadzu) in air atmosphere. X-ray diffraction (XRD) data from the samples were recorded with PC1800 diffractometer using Cu Kα radiation. The Brunauer–Emmett–Teller (BET) surface area, pore size, the distribution of pore size of MWNT, and NiO/MWNT samples were measured by nitrogen adsorption/desorption measurements using Autosorb 1-C, Quantachrome. Finally, the magnetic behavior of pure and NiO-decorated MWNT was obtained using vibrating sample magnetometer (VSM) at room temperature.
Results and discussion
TEM micrographs of pristine MWNTs (P-MWNTs) and functionalized MWNTs (FMWNTs) are shown in Fig. 1. Based on these images, the inner diameter of P-MWNTs is in the range of 5–20 nm, and the outer diameter is in the range of 40–60 nm and almost the closed end caps of P-MWNTs are revealed. As shown in Fig. 1b, almost all FMWNTs opened after acid treatment.
NiO/FMWNT nanoparticles were prepared with two different nickel oxide loadings (15 and 80 %) using the wet chemical method. Figure 2 shows TEM image of decorated MWNT with 15 and 80 wt% nickel oxide particles (15 % NiO-FWMNT, 80 % NiO-FMWNT). As seen, the NiO nanoparticles closely attached to the FMWNTs surface. The average size of the nickel oxides is in the range of 5–60 nm. In addition, the NiO particles are spread homogenously on the outer surface of MWNTs. Based on the figures, the crystallites of nickel oxide of 80 % NiO/FMWNT sample become larger than that of the 15 % NiO/FMWNT. Furthermore, at low nickel oxide, loading the NiO particles precipitates at the ends of CNTs; unlike the 80 % NiO/FMWNT, nearly all FMWNTs are covered with nickel oxide. The uniform deposition of NiO nanoparticles on FWMNT can be the results of uniform functional sites on the outer surface of all CNTs, which can be created in the acid treatment.
To investigate the thermal stability of P-MWNT, FMWNT, and 15 and 80 % NiO/FMWNT, the TGA measurement was performed in air atmosphere and at temperature range of 25–1000 °C. Figure 3 shows the TGA graph of all samples in air atmosphere. The degradation of P-MWNT occurs at one step in a temperature range of 610–710 °C. The weight loss below 100 °C is related to water loss, between 150 and 400 °C is evolved from carboxyl groups, and weight loss at higher 400 °C can be due to the loss of oxygen atom that tightly bonds to the surface of the MWNTs or non-graphitic carbonaceous species. According to TGA graph, there is no significant weight loss before 500 °C in the P-MWNTs. This is concluded that the presence of a significant amount of amorphous carbon and carboxyl group rules out. Besides, the obtained results of TGA and DTG curves are summarized in Table 1. The temperature at initial weight loss (T 0), maximum weight loss rate I, the final weight loss (T f), and residue at 800 °C are shown in Table 1. As seen from the data, the T 0 and T m appear at higher temperature when the P-MWNT was acid treated for 4 h. It is related to initial oxidation stage of FMWNT. The oxidant starts to attack active sites already existing on the MWNTs, such as CH2 and CH groups, so the thermal stability of FMWNTs improves. Moreover, the acid treatment does not deteriorate the thermal stability of MWNT, because the acid treatment cannot create the defect sites. Therefore, the thermal stability of P-MWNT using acid treatment is improved. Besides, the weight loss between 150 and 400 °C is the amount of carboxyl group that equals to 2.5 w% of total weight loss of the FMWNT sample.
Figure 4 shows the TGA curve and the derivative curve of the decomposition of Ni(NO3)2·6H2O in air atmosphere. As shown, at 225 °C, water completely evaporates, and nickel nitrate starts to decompose to nickel oxide at higher temperature than 280 °C. Therefore, the calcination should be greater than 280 °C. It can be seen that at 350 °C, a 72 % weight loss is detected which is related to decomposition of Ni(NO3)2·6H2O to NiO. Therefore, the calcination temperature of Ni(NO3)2·6H2O is selected at 350 °C for 2 h.
Figure 5 shows the TGA and DTG graphs of 80 % NiO/FMWNT. The oxidation of 80 % NiO/FMWNT has a single step, and it is observed that T 0 and T m occur at about 400 and 600 °C. Therefore, it is concluded that nearly all FMWNTs are coated with NiO nanoparticles. These observations are presented at TEM micrograph from Fig. 2. The residue mass at 800 °C is 80 wt% for NiO/FMWNT. It is determined that the content of NiO in NiO/FMWNT is about 80 %. According to Table 1, all temperatures T 0, T m, and T f decrease when the weight percent of NiO nanoparticles on the surface of FMWNTs increase. The reason of this effect is related to increase the amount of oxygen on the surface of FWMNT.
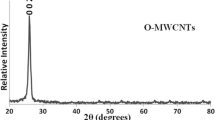
The XRD patterns of P-MWNT and 80 % NiO-FMWNT are shown in Fig. 6. The peaks at 26.2°, 44.2°, and 53.8° are observed in the three diffractions of graphite. By comparing the intensity of the peaks, no apparent difference is observed. It indicates that the graphene sheets of the MWNTs’ supports have not been destroyed after acid treatment and the loading of NiO species. The diffraction peaks at 37.24°, 43.3°, 62.87°, 62.91°, 75.3°, and 79.3° correspond to NiO.
To create active sites to anchor NiO nanoparticles on the surface of MWNTs, the MWNTs were oxidized and were investigated by FTIR, as shown in Fig. 7. P-MWNT shows very low intensity at around 3440, 1711 corresponding to OH and C = O. OH peak is related to water molecules on the carbon in the CNTs. In the case of FMWNT, there is an additional peak observed at 1719 cm−1 which is due to the C = O stretching frequency, indicating that the presence of carboxylic group is created during the oxidation of MWNTs. These characteristic bonds appear with significantly higher intensity. This is attributed to increase the number of carboxylic groups which has been successfully attached to the surface of MWNT after acid treatment.
The FTIR spectrum of NiO-FMWNT shows the peaks with very low intensity at around 3440 and 1711 cm−1. This effect is related to NiO particles anchor to MWNT using carboxylic group on the surface of FMWNTs. As it is indicated by TGA results, the weight loss of NiO-FMWNT at temperature smaller than 400 °C is not detected. It is noted that all functional groups are used to NiO nanoparticles attached to the outer surface of FMWNTs.
Figure 8 shows the magnetic behavior of P-MWNT and 80 % NiO/FWMNT samples derived by VSM analysis at room temperature. Hysteresis loops for P-MWNT and 80 % NiO/FWMNT are shown. The curves of two samples are symmetric around H = 0. The coercivity (H c) is determined to 198.3 Oe for P-MWNT, and 182.7 Oe for 80 % NiO/FWMNT. Besides, the saturation magnetization (M s) of P-MWNT is about 0.385 emu/g. The saturation magnetization of 80 % NiO/FWMNT is lower than that of P-MWNT.
The nitrogen adsorption at 77 K is powerful and convenient method to measure the specific surface area, pore size distribution, and pore volume of porous materials. The N2 adsorption–desorption isotherm of P-MWNT and 15 % NiO/FMWNT is shown in Fig. 9. As shown, sharp increase in N2 adsorption below p/p 0 = 0.05 can be detected. This variation can be attributed to the micropores existed in MWNT [14, 23]. In addition, slow increasing trend in the range of 0.05–0.7 during adsorption curve demonstrates the presence of mesopore structures in the both samples [12]. Slow rising adsorption of nitrogen on MWNT is related to the multilayer adsorption reaction on the surface of MWNT. It is concluded to the formation of N2 film on the surface of MWNT and capillary phenomenon at the mesopores site.
The adsorption and desorption curves show an overlap in the p/p 0 range of 0.05–0.5. Besides, in the p/p 0 range of 0.6–0.99, the adsorption hysteresis loop can be observed.
The BET surface areas of P-MWNT and 15 % NiO/FWMNT are 50 and 52 m2/g, respectively. The total pore volume and average pore diameters are 3.33 × 10−2 cc/g, 2.0 nm for P-MWNT and 7.43 × 10−2 cc/g, 6.9 nm for 15 % NiO/FMWNT. Comparison of the BET data shows the slight increase in specific surface areas and porosities whenever the NiO nanoparticles deposited on the surface of CNT. It can be attributed to the role of NiO nanoparticles to enhance the surface roughness and opening the ends of MWNTs rather than P-MWNT.
Conclusions
In summary, the formation of NiO nanoparticles on MWNTs with the wet chemical method was studied. The chemical impregnation process led to homogenously dispersed NiO nanoparticles on the surface of FMWNTs, and the particles were found to possess an average size of 5–60 nm. The presence of nickel oxide on the surface of MWNTs causes to increase the surface roughness. It could be proved by the results of BET data as the slight increment in the specific surface areas and porosities by the presence of the NiO nanoparticles on the surface of CNT.
References
Ahmadpoor, F., Zebarjad, S.M., Janghorban, K.: Decoration of multi-walled carbon nanotubes with silver nanoparticles and investigation on its colloid stability. Mater. Chem. Phys. 139, 113 (2013)
Ajayan, P.M., Zhou, O.Z.: Applications of carbon nanotubes. Top. Appl. Phys. 80, 391 (2001)
Baughman, R.H., Zakhidov, A.A., De Heer, W.A.: Carbon nanotubes–the route toward applications. Science 297, 787 (2002)
Bittencourt, C., Felten, A., Ghijsen, J., Pireaux, J.J., Drube, W., Erni, R., Van Tendeloo, G.: Decorating carbon nanotubes with nickel nanoparticles. Chem. Phys. Lett. 436, 368 (2007)
Capobianchi, A., Foglia, S., Imperatori, P., Notargiacomo, A., Giammatteo, M., Del Buono, T., Palange, E.: Controlled filling and external cleaning of multi-wall carbon nanotubes using a wet chemical method. Carbon. 45, 2205 (2007)
Chen, Q., Xu, R., Yu, D.: Multiwalled carbon nanotube/polybenzoxazine nanocomposites: preparation, characterization and properties. Polymer 47, 7711 (2006)
Chin, K.C., Gohel, A., Elim, H.I., Ji, W., Chong, G.L., Lim, K.Y., Sow, H., Wee, A.T.S.: Optical limiting properties of amorphous SixNy and SiC coated carbon nanotubes. Chem. Phys. Lett. 383, 72 (2004)
Correa-Duarte, M.A., Grzelczak, M., Salgueiriño-Maceira, V., Giersig, M., Liz-Marzá, N.L.M., Farle, M., Sierazdki, K., Diaz, R.: Alignment of carbon nanotubes under low magnetic fields through attachment of magnetic nanoparticles. J. Phys. Chem. B. 109(41), 19060 (2005)
Dong, Z., Ma, K., He, J., Wang, J., Li, R., Ma, J.: Decorating carbon nanotubes with cobalt nanoparticles. Mat. Lett. 62, 4059 (2008)
Fantini, M.C.A., Ferreira, F.F., Gorenstein, A.: Theoretical and experimental results on Au–NiO and Au–CoO electrochromic composite films. Solid State Ionics. 152–153, 867 (2002)
Gao, B., Yuan, C-Z., Su, L.H., Chen, L., Zhang, X-G.: Nickel oxide coated on ultrasonically pretreated carbon nanotubes for supercapacitor. J. Solid State Electrochem. 13, 1251 (2009)
Gregg, S.J., Sing, K.S.W.: Adsorption, surface area and porosity. Academic Press, London (1982)
Iijima, S.: Helical microtubules of graphitic carbon. Nature 354, 56 (1991)
Jaroniec, M., Kaneko, K.: Physicochemical foundations for characterization of adsorbents by using high-resolution comparative plots. Langmuir 13, 6589 (1997)
Kahattha, C., Woointranont, P., Chodjarusawad. T., Pecharapa. W.: Study of acid-treated multiwall carbon nanotubes by electron microscopy and raman spectroscopy. J. Microsc. Soc. Thai. 24, 133 (2010)
Li, Z., Pan, Z., Dai, S.: Nitrogen adsorption characterization of aligned multiwalled carbon nanotubes and their acid modification. J. Colloid. Interface Sci. 277, 35 (2004)
Mattei, G., Mazzoldi, P., Post, M.L., Buso, D., Guglielmi, M., Martucci, A.: Cookie-like Au/NiO nanoparticles with optical gas-sensing properties. Adv. Mater. 19, 561 (2007)
Oh-Kil, K., Jongtae, J., Jeffrey, W.B., Steven, K., Pehr, P.E., Leonard, J.B.: Decoration of carbon nanotubes with iron oxide. J. Am. Chem. Soc. 125, 4426 (2003)
Oymak, H., Erkoc, S.: Titanium coverage on a single-wall carbon nanotube: molecular dynamics simulations. Chem. Phys. 300, 277 (2004)
Park, J., Kang, E., Son, S.U., Park. H.M., Lee, M.K., Kim, J., Kim, K.W., Noh, H.J., Park, J.H., Bae, C.J., Park, J.G., Hyeon, T.: Monodisperse nanoparticles of Ni and NiO: synthesis, characterization, self-assembled super lattices, and catalytic applications in the suzuki coupling reaction. Adv. Mater. 17, 429 (2005)
Qiao, H., Wu, N., Huang, F., Cai, Y., Wei, Q.: Solvothermal synthesis of NiO/C hybrid microspheres as Li-intercalation electrode material. Mater. Lett. 64, 1022 (2010)
Robertson, N., Mcgowan, C.A.: A comparison of potential molecular wires as components for molecular electronics. Chem. Soc. Rev. 32, 96 (2003)
Sing, K.S.W., Evertt, D.H., Haul, R.A.W., Moscou, L., Pierotti, R.A., Rouquerol, J., Siemieniewska, T.: Reporting physisorption data for gas/solid systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 57, 603 (1985)
Song, W., Zheng, Z., Lu, H. and Wang, X.,: Incorporation of multi-walled carbon nanotubes into biodegradable telechelic prepolymers. Macromol. Chem. Phys. 209, 315 (2008)
Watts, P.C.P., Hsu, W.K., Randall, D.P., Kotzeva, V., Chen, G.Z.: Fe-filled carbon nanotubes: nano-electromagnetic inductors. Chem. Mater. 14, 4505 (2002)
Wu, Y.H., Qiao, P.W., Qiu, J.J., Chong, T.C., Low, T.S.: Magnetic nanostructures grown on vertically aligned carbon nanotube templates. Nano. Lett. 2, 161 (2002)
Zhang, Q., Zhu, M., Zhang, Q., Li, Y., Wang, H.: The formation of magnetite nanoparticles on the sidewalls of multi-walled carbon nanotubes. Comp. Sci. Technol. 69, 633 (2009)
Zhu, J., Peng, H., Rodriguez-Macias, F., Margrave, J.L., Khabashesku, V.N., Imam, A.N., Lozano, K., Barrera, E.V.: Reinforcing epoxy polymer composites through covalent integration of functionalized nanotubes. Adv. Funct. Mater. 14, 643 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sahebian, S., Zebarjad, S., Vahdati Khaki, J. et al. The decoration of multi-walled carbon nanotubes with nickel oxide nanoparticles using chemical method. Int Nano Lett 6, 183–190 (2016). https://doi.org/10.1007/s40089-016-0185-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-016-0185-8