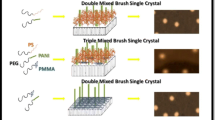
Abstract
Polymer brushes due to their high sensitivity to environmental changes are the best and newest means for developing the responsive materials. Polymer nano-brushes consisting various surface morphologies and uniformly distributed amorphous grafted chains were synthesized via single-crystal growth procedure. Poly(ethylene glycol)-b-polystyrene (PEG-b-PS) and poly(ethylene glycol)-b-poly(methyl methacrylate) (PEG-b-PMMA) block copolymers were prepared by atom transfer radical polymerization (ATRP). On the basis of various height differences, phase regions were detectable through atomic force microscopy (AFM NanoscopeIII). The novelty of this work is developing and characterizing the random and intermediate single-co-crystals. Besides, some other sorts of brush-covered single crystals like homo-brush and matrix-dispersed mixed-brushes were involved just for comparing the distinct morphologies. The intermediate (neither matrix-dispersed nor random) single-co-crystals were detectable through their thickness fluctuations in AFM height profiles. On the contrary, the random single-co-crystals were verified through comparing with their corresponding homopolymer and homo-brush single crystals. The growth fronts of (120), (240), (200) and (040) were detected by electron diffraction of transmission electron microscope.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Property control of materials has always been a main purpose of scientists. Modification of the properties is achievable through manipulation of structure, composition, and surface [1, 2]. Recently, for high sensitivity of polymer and bio-polymer systems, polymer brushes have drawn enormous attraction. The polymer nano-brushes are capable of changing their conformation against the environmental conditions, and this is their benefit for surface modification.
To graft chains to substrates, several methods were introduced by physical adsorption, grafting to, and grafting from approaches [3–5]. Mentioned approaches are not able to control the uniformity of tethering density [6] and chain length of the tethered polymers. The crystal surface engineering method to generate tethered chains on a single-crystal basal surface using crystalline–amorphous block copolymers is the third way [7].
An especial heterogeneous nucleation is entitled self-seeding explaining the nucleation begun through chemically identical seeds. Self-seeding lead to similar crystal sizes specifically in dilute growth conditions. To some extent, the self-nucleation process can control the final crystal size by controlling the population of the seeds through the self-seeding temperature [8]. The thickness of the poly(ethylene glycol) (PEG) crystal layer in the block copolymer single crystals changes with the crystallization temperature, and this affects the conformation of the amorphous chains. Since amorphous chains are tethered to the crystal layer surface, they are considered to be polymer brushes. Demanded block copolymers to fabricate single crystals with predetermined molecular weight could be prepared by living polymerization methods (e.g., ionic or radical polymerization). Because for developing homogeneous and uniform amorphous brushes the primary constituent chains should resemble each other length wise (i.e., narrow polydispersity). Uniform brushes developed by this approach cannot be realized by traditional methods for polymer brush fabrication. This method forms a film which is attached to a substrate by covalent bonding. When a long block such as atactic polystyrene (PS) chain is attached to a crystalline PEG chain, the big chain ends are excluded from the crystal lattice and they accumulate onto the crystalline substrate surface developing polymer brushes [7, 9]. Polymer mixed-brushes having various surface morphologies, just like homo-brush single crystals, are composed of sandwich structure [10, 11], i.e., a PEG nano-layer sandwiched between two amorphous nano-layers.
In this research, we have developed intermediate and random single-co-crystals comprising homo and block copolymers with common crystalline blocks. Matrix-dispersed single-co-crystals and mixed-brushes in solution and melt state were previously reported by our research group [11–13]. They are also recalled here to make some comparisons. Developed polymer brushes could be used in surface modification against pH, drug delivery, and biosensors. Our research group is working on the single crystals with conducting polymer brushes on the surface. The more novel results will be reported in the frame of future works. These kinds of single crystals could be employed in polymer solar cells and thin film transistors.
Experimental
Material synthesis
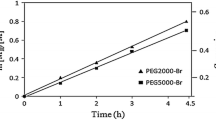
The homopolymers of PEG (Sigma-Aldrich) were dried by azeotropic distillation with anhydrous toluene (98 %) before utilization. Traces of residual toluene were removed under vacuum. The PEG-Br macroinitiator was synthesized according to reported data [14]. The diblock copolymers of PEG-b-PS and PEG-b-PMMA were synthesized by solution polymerization in chlorobenzene via atom transfer radical polymerization (ATRP). In an experiment, an ATRP reactor was charged with desired ratio of monomer, PEG-Br macroinitiator, CuBr, 2,2′-bipyridine, and chlorobenzene with the ratio of [monomer]0/[PEG-Br]0/[CuBr]0/[bipy]0/[chlorobenzene]0) = 250/1/1/3/65. After purging with high-purity nitrogen for half an hour, the valves and inlet were closed, and the reactor was immersed into a thermostatic oil bath at 110 and 65 °C for styrene (St) and methyl methacrylate (MMA) polymerization, respectively, under rigorous stirring. After passing desired time, the reactor was withdrawn and cooled to room temperature. The reaction mixture was diluted with tetrahydrofurane (THF) and then was filtered through a neutral Al2O3 column to remove the catalyst and, subsequently, precipitated with petroleum ether. The resulted diblock copolymers were dried under vacuum overnight at room temperature. Detailed synthesis procedures were described elsewhere [15–17]. The molecular weight (M n) of PEG5000-PMMA block copolymers vs. conversion and the molecular weight of PEG5000-PS block copolymers vs. time are plotted in Fig. 1. These linear graphs could indicate the first-order kinetic of the polymerization of St and MMA. Each data points were obtained from 1H NMR analyses of PEG-b-PS and PEG-b-PMMA diblock copolymers. The polydispersity index (PDI) of synthesized chains taken from gel permeation chromatography (GPC) was narrow (1.11–1.21) and, consequently, the length of tethered nano-brushes onto the PEG substrate was uniform. By comparing the differential scanning calorimetry (DSC) thermograms of PEG5000-b-PS10000 single-crystal mats and the bulk of the corresponding diblock copolymers, it was found that the melting temperature (T m) of single-crystal mats was higher than that of bulk of diblock copolymers (55 vs. 52 °C). It was associated with higher crystallinity and intrinsic order in the structure of single crystals. Figure 2 depicts the mentioned DSC thermograms.
Single-crystal growth
The self-seeding approach employed for growth of PEG-b-PS diblock copolymer single crystals is described elsewhere [8]. Solution crystallization was carried out with a dilute concentration of 0.005–0.02 wt % in amyl acetate. The sample was put into the cell tube and heated to above the dissolution temperature (T d = 65 °C) for several minutes. Then, cell tube was switched to a 0 °C bath lasting 5 h for conducting primary crystallization, and then immersed into a given self-seeding temperature oil bath [17], and kept for 20 min. The cell tube was then quickly transferred into a desired crystallization temperature (T c = 23–30 °C) bath and kept for 3 days.
Apparatus
The synthesized block copolymers were analyzed by 1H NMR spectroscopy on a Bruker (Avance DPX) spectrometer working at 400 MHz. The PDIs of fostered polymers were determined by GPC on a WATER 1515 (USA) gel permeation chromatography instrument with a set of HT3, HT4, and HT5, μ-styragel columns with DMF and THF as eluents for PS and poly(methyl methacrylate) (PMMA), respectively (1.0 mL/min), at 35 °C. A set of monodisperse polystyrene standards were utilized for calibration. Differential scanning calorimetry (DSC, NETZSCH, F3 Maia200) experiments were carried out to study the thermal behaviors. The total thickness of single crystals and their surface morphologies were identified with the help of an AFM, NanoscopeIIIA. Olympus MPG3 microscope was mainly used to take a first look on the single-crystal population distribution and the sample uniformity. Single crystals were also observed under a transmission electron microscope (TEM, EM 208 Philips) equipped with electron diffraction (ED) technique having an accelerating voltage of 100 keV.
Results and discussion
In this work, random and intermediate single-co-crystals have been prepared in a dilute system including crystalline homopolymers and crystalline–amorphous diblock copolymers by self-seeding method. Moreover, the polymer mixed-brushes as well as homo-brushes have been recalled. A single-co-crystal is a uniform crystalline structure having two or more crystallizable constituents. Totally, in single-co-crystal growth systems there were three various surface morphologies divided into matrix-dispersed, intermediate and random morphologies. In matrix-disperse morphology [11], the domain size of disperses were detectable in matrix phase. The domain size of dispersed patches did not vary with crystallization temperature significantly. They decreased with M PSn and M PMMAn enhancement, however. The cause for this phenomenon that some of co-crystals are random and some others are matrix-dispersed could be attributed to the partially high PDI in our systems.
Polymer brushes from single co-crystallization of PEG5000-b-PS10000/PEG5000 at crystallization temperature of 32 °C are depicted in Fig. 3a. This surface morphology is an arrangement which is neither matrix-disperse nor random cocrystal. We called them intermediate single-co-crystals. Respective AFM height profile of marked parts as well as electron diffraction (ED) pattern of TEM are depicted in Fig. 3b, c, respectively. The growth fronts were (120), (240), (200), and (040). Height profile proves that the thickness of single crystals varies between 11.40 nm for homo-PEG5000 and 19.20 nm for total thickness of PS10000-covered PEG. In this kind of single crystals, PS-covered and bared-PEG areas are mixed together and small areas are detectable by AFM.
Figure 4 depicts the surface morphology of PEG5000-b-PMMA8700/PEG5000 random single-co-crystal at T c = 23 °C. The random single-co-crystals are constructed from homo and copolymer chains, simultaneously. In these systems, the domain sizes may be too infinitesimal and, consequently, their detection was beyond analysis accuracy of AFM. The substantial difference between these random single-co-crystals and corresponding homo-brush single crystals was in the total height and especially in the PEG substrate thickness, which were higher for random single-co-crystals. The height profiles of single crystals grown at T c = 23 °C including PEG5000 (10.33 nm) single crystal, random single-co-crystal of PEG5000-b-PMMA17100/PEG5000 (17.36 nm), and homo-brush single crystal of PEG5000-b-PMMA17100 (12.42 nm) are depicted in Fig. 5a–c, respectively. These differences could be associated with decrease in the segmental density and, consequently, the osmotic pressure (pressure exerted by a tethered chain on the surface of single-crystal substrate to provide its required surface area to be expanded) of tethered chains. Hence, the respective osmotic pressure on the substrate surface will diminish. Randomly addition of homo-PEG chains into the assembly of diblock copolymer chains is entitled random cocrystallization. In fact, it is replacing a percentage of diblock copolymers with homopolymers in the structure of single crystal. Through mentioned method, the tethering density of grafted chains on the substrate could be reduced without altering the molecular weights and crystallization temperature. In the random single-co-crystal systems, the only effective factor which could raise the thickness of substrate is disapproach of tethered brushes from each other. Crystallization which occurs randomly could be considered as a way to increase the substrate thickness despite the fact that the tethering density is getting reduced. It is of interests that in conventional homo-brush single-crystal growth systems, the trend of substrate thickness and tethering density is the same. As an instance, when the substrate thickness increases via crystallization temperature, the chains tend to gain more stretched conformations. Where the folding spaces are constant, the grafted chains on the substrate surface approach together, and this is the very meaning of tethering density enhancement via rising the substrate thickness. Actually, this disapproach has primarily allowed the substrate to increase its height through decreasing of osmotic pressure on it. The novelty of this work is developing of random and intermediate single-co-crystals. Previously, matrix-dispersed single-co-crystals were introduced and investigated [11].
The height profiles obtained via scanning the crystals crystallized at T c = 23 °C in amyl acetate dilute solution; homopolymer single crystal of PEG5000, thickness: 10.33 nm, lateral size: 6.41 μm (a); random single-co-crystal of PEG5000-b-PMMA17100/PEG5000, thickness: 17.36 nm, lateral size: 5.97 μm (b); homo-brush single crystal of PEG5000-b-PMMA17100, thickness: 12.42 nm, lateral size: 5.86 μm (c). The weight ratio of PEG5000-b-PMMA17100/PEG5000 was 50/50 for cocrystallization
Single-crystal method is one of best ways to prepare uniform polymer brushes with high level of control on effective parameters. Indeed, in mixed-brush growth systems, the quality of solvent for amorphous blocks in solution as well as the behavior of different chains grafted on the substrate surface lead to varying arrangements of polymer brushes. Amyl acetate at growth condition is very good and partially poor for PS and PMMA blocks in respect [18, 19]. The interaction between PMMA brushes and PEG substrate is attractive. However, the interaction between PS brushes and PEG substrate is repulsive [19, 20]. These two kinds of interactions conduce to different conformations for amorphous brushes which form various phase regions on the substrate surface. That is why in mixed-brush single crystals, the morphologies were matrix-dispersed [12] and co-continuous in solution and melt conditions [13], respectively. In addition to matrix-dispersed [12] and co-continuous mixed-brushes [13], matrix-dispersed, co-continuous [11], random and intermediate single-co-crystals, the homo-brush single crystals were developed in solution and melt states by our research group. These single crystals had smooth and homogeneous surfaces. In Fig. 6a, b AFM height images of homo-brush single crystals of PEG5000-b-PMMA8700 at T c = 28 °C and PEG5000-b-PS4600 at T c = 23 °C are illustrated, respectively. Figure 7 depicts AFM height image and height profile of PEG5000-b-PMMA13100/PEG5000-b-PS4600 matrix-dispersed mixed-brush single crystal grown at T c = 23 °C.
In homo-brushes as well as polymer mixed-brushes, by elevating the crystallization temperature, the total height and substrate thickness of single crystals in different phases were raised. This increase is associated with the substrate thickness increase as well as amorphous brushes thickening. Changing the crystallization temperature from 23 to 32 °C for PEG5000-b-PS4600/PEG5000-b-PMMA8700 mixed-brush morphologies, the PS phase thickness reached from 14.2 to 16.8 nm, whereas the thickness of PMMA dispersed phase increased from 10.0 to 11.9 nm, respectively. Similarly, altering the crystallization temperature from 28 to 32 °C for PEG5000-b-PS14800/PEG5000 cocrystal morphologies, the PS phase thickness varied from 20.8 to 21.8 nm, whereas the thickness of PEG bared phase region increased from 11.0 to 11.5 nm, respectively.
Total thicknesses of grown single crystals were obtained by AFM. Equations (1)–(3) [7, 9] are used to determine the PEG crystalline substrate thickness.
where \(v_{\text{CRYST}}\) is volume percentage of PEG substrate; \(M_{\text{n}}^{\text{CRYST}}\) and \(M_{\text{n}}^{\text{AMORPH}}\) denote molecular weight of PEG (=5000 g/mol) and amorphous blocks. We assume that the density of the two PS and PMMA block layers are identical to those of the amorphous PS and PMMA bulks (\(\rho_{\text{AM}}\) is 1.052 and 1.190 g/cm−3 for PS and PMMA, respectively). The densities of the crystalline (\(\rho^{\text{c}}_{\text{CRYST}}\)) and amorphous (\(\rho^{\text{a}}_{\text{CRYST}}\)) PEG are identical to the bulk densities (1.239 and 1.124 g/cm−3, respectively) at room temperature [18, 21]. Finally, \(w^{\text{c}}\) is the crystallinity of PEG in single crystal which in this system possesses 95 % crystallinity based on the DSC study on the single-crystal mats.
Tethering density (σ) is defined as the number of tethering points in a unit area, and it is an important parameter to describe polymer brushes. Tethering density could be calculated with Eq. (4) [9]. Here, \(N_{0}\) shows the Avogadro number (6.02 × 1023 mol−1).
Because the chain conformation is dependent on the molecular weight and surrounding environmental conditions such as temperature, solvent quality and other factors, the reduced tethering density (\(\tilde{\sigma }\)) is more generally used instead of tethering density, σ. Reduced tethering density is defined as \(\tilde{\sigma } = \sigma \pi R_{\text{g}}^{2}\) [22], where \(R_{\text{g}}\) is the radius of gyration. Therefore, the physical meaning of \(\tilde{\sigma }\) is the number of tethered chains in the area occupied by one unperturbed polymer chain. Based on the reduced tethering density, there are three regimes for polymer brushes which are divided in non-interaction regime, crossover regime, and highly stretched regime [7, 16].
In mixed-brush together with homopolymer and copolymer co-crystallization systems, the portion of dispersed or patched phase areas decreases via escalating of molecular weight of amorphous tethered chains on the single-crystal substrate.
Another parameter investigated in this research is temperature and its fluctuations. Crystallization sections were done in isothermal condition to obtain ideal single crystals, because temperature fluctuation results in the structures with different thicknesses in its different parts. During the crystallization, the temperature changes were maintained below ±0.2 °C.
All single crystals are not monolayer and ideal. Some parameters such as crystallization temperature fluctuations, high concentration, extreme deviation from optimized self-seeding temperature and so on could conduce to multilayer and non-ideal crystals. Some instances of this type of crystals are represented in Fig. 8. Presence of grafted chains as polymer brushes on the single-crystal surface can in turn help suppressing the mentioned deviation from ideal and monolayer state.
The other effect of crystallization temperature on growth systems is its relationship with thickness. Investigating the obtained AFM height profiles, as mentioned previously, we found out that via elevation of crystallization temperature, the thickness of all phase regions increased. This height soaring could be ascribed to substrate and brushes thickening simultaneously, because the grafted chains will get closer together. In detail, the thickness enhancement of crystalline substrate could be related to the tendency of PEG chains to reach to the more stretched state to reduce the free energy [23] and the thickening of amorphous tethered brushes is for their tendency to gain more extended conformations, and neutralize the overlapping of chains in the vicinity of others (which could happen due to approach of tethered chain on the basis of folding numbers decrease). Approaching of brushes to each other means that tethering density is on the rise, because the tethering density is the number of grafted chains per surface area. Therefore, through elevating the crystallization temperature, total thickness and, consequently, tethering density will increase in various regions of polymer brushes. As an instance, for PEG5000-b-PS4600 homo-brush single crystal when the crystallization temperature reached from 23 to 30 °C, dtotal, dPEG, and subsequently dPS changed from 13.9 to 15.80 nm, from 6.5 to 7.2 nm, and finally from 3.7 to 4.3 nm, respectively. The respective tethering densities were 0.47 and 0.53 nm−2, in respect.
In homo-brush as well as single-co-crystals, through increasing the molecular weight of amorphous blocks, the total thickness raised while the crystalline substrate thickness decreased. Thickening of amorphous layer lies in the heightening of brushes as well as their stretching so as to overcome the overlap of chains in their vicinity. Subsequently, to provide their coverage surface area, they will cause the substrate to decrease its thickness by more folding. So, the tethered chains on the substrate surface will diverge from each other.
Conclusions
Polymer brushes having various morphologies were created through crystallization of homo-PEG and crystalline–amorphous chains of PEG-b-PS as well as PEG-b-PMMA in a dilute solution. Two novel types of single-co-crystals including intermediate and random were introduced and characterized. In height profile of PEG5000-b-PS10000/PEG5000 intermediate (neither matrix-dispersed nor random) single-co-crystals, the thickness of single crystals varied between 11.40 nm for homo-PEG5000 and 19.20 nm for total thickness of PS10000-covered PEG. The thicknesses of PEG5000 (10.33 nm) single crystal, random single-co-crystal of PEG5000-b-PMMA17100/PEG5000 (17.36 nm), and homo-brush single crystal of PEG5000-b-PMMA17100 (12.42 nm) were compared to detect the random cocrystallization. Besides, with elevation of crystallization temperature, the thickness and, subsequently, tethering density of different phase regions were on the rise.
References
Lahann, J., Mitragotri, S., Tran, T.N., Kaido, H., Sundaram, J., Choi, I.S., Hoffer, S., Somorjal, G.A., Langer, R.: Reversible switching of surfaces. Science 299, 371–374 (2003)
Russel, T.P.: Surface-responsive materials. Science 297, 964–967 (2002)
Milner, S.T.: Polymer brushes. Science 251, 905–914 (1991)
Halperin, A., Tirrell, M., Lodge, T.P.: Tethered chains in polymer microstructures. Adv. Polym. Sci. 100, 31–37 (1992)
Mansky, P., Liu, Y., Huang, E., Russel, T.P., Hawker, C.J.: Controlling polymer-surface interactions with random copolymer brushes. Science 275, 1458–1460 (1997)
Taunton, H.J., Toprakcioglu, C., Fetters, L.J., Klein, J.: Forces between surfaces bearing terminally anchored polymer chains in good solvents. Nature (London). 332, 712–713 (1988)
Chen, W.Y.; Zheng, J.X.; Cheng, S.Z.D.; Li, C.Y.; Huang, P.; Zhu, L.; Xiong, H.; Ge, Q.; Guo, Y.; Quirk, R.P.; Lotz, B.; Deng, L.; Wu, C.; Thomas, E.L.; Onset of Tethered Chain Overcrowding, Phys. Rev. Lett., 93, 028301 (1–4) (2004)
Lotz, B., Kovacs, A.J.: Propriétés des Copolymères Biséquencés Polyoxyéthylène-Polystyrène. Colloid Polym. Sci. 209, 97–114 (1966)
Zheng, J.X., Xiong, H., Chen, W.Y., Lee, K., Van Horn, R.M., Quirk, R.P., Lotz, B., Thomas, E.L., Shi, A.-C., Cheng, S.Z.D.: Onsets of tethered chain overcrowding and highly stretched brush regime via crystalline amorphous diblock copolymers. Macromolecules 39, 641–650 (2006)
Chen, W.Y., Li, C.Y., Zheng, J.X., Huang, P., Zhu, L., Ge, Q., Quirk, R.P., Lotz, B., Deng, L., Wu, C., Thomas, E.L., Cheng, S.Z.D.: Chemically shielded poly(ethylene oxide) single crystal growth and construction of channel-wire arrays with chemical and geometric recognitions on a submicrometer scale. Macromolecules 37, 5292–5299 (2004)
Agbolaghi, S., Abbasi, F., Abbaspoor, S., Alizadeh-Osgouei, M.: Self-designed surfaces via single-co-crystallization of homopolymer and diblock copolymers in various growth conditions. Eur. Polymer J. 66, 108–118 (2015)
Abbaspoor, S., Abbasi, F., Agbolaghi, S.: A novel approach to prepare polymer mixed-brushes via single crystal surface patterning. RSC Adv. 4, 17071–17082 (2014)
Agbolaghi, S., Alizadeh-Osgouei, M., Abbaspoor, S., Abbasi, F.: Self-assembling nano mixed-brushes having cocontinuous surface morphology by melt growing single crystals and comparison with solution patterned leopard-skin surface morphology. RSC Adv. 5, 1538–1548 (2015)
Jankova, K., Chen, X.Y., Kops, J., Batsberg, W.: Synthesis of amphiphilic PS-b-PEG-b-PS by atom transfer radical polymerization. Macromolecules 31, 538–541 (1998)
Jankova, K., Chen, X.Y., Kops, J., Batsberg, W.: Controlled/living ATRP of styrene in the synthesis of amphiphilic diblock copolymers from a poly(ethylene glycol) macroinitiator. Polym. Bull. 42, 153–158 (1999)
Agbolaghi, S., Abbasi, F., Jalili, K.: Nascent lateral habits of solution crystallization of Poly(ethylene glycol)-block-Polystyrene diblock copolymers. J. Polym. Res. 21(380), 1–11 (2014)
Agbolaghi, S., Abbasi, F., Abbaspoor, S.: Epitaxial single crystal surface patterning and study of physical and chemical environmental effects on crystal growth. Colloid Polym. Sci. 292, 1375–1383 (2014)
Brandup, J., Immergut, E.H.: Polymer Handbook. John Wiley and Sons, Inc., Publication (1975)
Van Horn, R.M., Zheng, J.X., Sun, H.-J., Hsiao, M.-S., Zhang, W.-B., Dong, X.-H., Xu, J., Thomas, E.L., Lotz, B., Cheng, S.Z.D.: Solution crystallization behavior of crystalline-crystalline diblock copolymers of poly(ethylene oxide)-block-poly(ε-caprolactone). Macromolecules 43(14), 6113–6119 (2010)
Chen, Y.; Single crystal engineering of amorphous-crystalline block copolymers crystallization, morphology and applications, Dissertation, University of Akron (2005)
Wunderlich, B.; Macromolecular physics Vol. 1: crystal structure, morphology, defects, Academic, New York (1973)
Kent, M.S.: A quantitative Study of Tethered Chains in Various Solution conditions Using Langmuir diblock copolymer Monolayers. Macromol. Rapid Commun. 21, 243–247 (2000)
Hamie, H.; Morphology and Thermal Behavior of Single Crystals of Polystyrene-Poly(ethylene oxide) Block Copolymers, Dissertation, University of Haute Alsace (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Agbolaghi, S., Abbaspoor, S. & Abbasi, F. Synthesis of polymer nano-brushes by self-seeding method and study of various morphologies by AFM. Int Nano Lett 6, 11–19 (2016). https://doi.org/10.1007/s40089-015-0166-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-015-0166-3