Abstract
Metal oxide nanoparticles offer great merits over controlling rheological, thermal, chemical and physical properties of solutions. The effectiveness of a nanoparticle to modify the properties of a fluid depends on its diffusive properties with respect to the fluid. In this study, rheological properties of aqueous fluids (i.e. water) were enhanced with the addition of CeO2 nanoparticles. This study was characterized by the outcomes of simulation and experimental results of nanofluids. The movement of nanoparticles in the fluidic media was simulated by a large-scale molecular thermal dynamic program (i.e. LAMMPS). The COMPASS force field was employed with smoothed particle hydrodynamic potential (SPH) and discrete particle dynamics potential (DPD). However, this study develops the understanding of how the rheological properties are affected due to the addition of nanoparticles in a fluid and the way DPD and SPH can be used for accurately estimating the rheological properties with Brownian effect. The rheological results of the simulation were confirmed by the convergence of the stress autocorrelation function, whereas experimental properties were measured using a rheometer. These rheological values of simulation were obtained and agreed within 5 % of the experimental values; they were identified and treated with a number of iterations and experimental tests. The results of the experiment and simulation show that 10 % CeO2 nanoparticles dispersion in water has a viscosity of 2.0–3.3 mPas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thermo-physical applications are creating an opportunity for a wide use of nanoparticles in fluids. The use of the CeO2 nanofluids for enhancing the thermo-physical and rheological properties has shown to be important in applications such as plate heat exchanger, fuel cells, tribology [1], heat transfer [2], coolant material [2], metal polishing, UV absorbent [3] and filters. The dependencies on nanoparticles are increasing due to their characteristics of high surface area to volume ratio, size, and stability where different nanoclusters show diffusive and thermo-physical enhancements [4–6]; likewise, CeO2 nanoclusters have capabilities of promoting different properties when dispersed in various fluids. It has been seen that CeO2 nanoparticle stability in water is low [1], whereas the stability can still be enhanced by modifying agents.
The improvement in dispersibility of CeO2 nanoparticles by surfactant Sorbitan monostearate in water demonstrated contribution to friction reduction [1, 7]. The application of this additive was able to reduce the friction coefficient of the water-based lubricant effectively, i.e. good for tribology applications. Later, wear scar depth observed with water-based CeO2 nanofluid presented less enhanced antiwear properties than the CeO2 modified with Sorbitan monostearate as investigated by Zhao et al. [7]. Later, ceria-based water nanofluids showed improvements in convective heat transfer applications; these nanofluids are capable of enhancing the heat transfer coefficient to about 39–40 % in a plate heat exchanger [2].
However, the CeO2 simulations were carried out to study the phase separation and particle aggregation, as Conesa investigated the CeO2 surface structural stability analysis simulations using molecular mechanics. Their simulations through molecular mechanics were 10 % in contrast with experimental results for bulk modulus of CeO2 [8].
Moreover, the metal oxides nanoparticles of silica (SiO2), ceria (CeO2), and alumina (Al2 O3) nanofluids at low concentrations with a particle size of 10 nm and 20 nm in pool boiling have been analysed. When these particles do not deposit on the wire, the nanofluid with metal oxides suspension increases critical heat flux by about 50 %, irrespective of the type of the oxide particle and its size [9].
Furthermore, Baudin et al. discussed the structural energies and surface properties over different orientations of the CeO2 by molecular dynamics using NPT ensemble [10–12].
Apart from nanofluid’s simulations, all these simulations that are mostly related to the structural analysis of CeO2 nanoparticles are beneficial and easily available. The CeO2 nanoparticles higher usages in different fluids make it important to simulate. Hence, due to the wide applications of CeO2 nanofluid, this study presents an investigation of the dispersion of CeO2 nanoparticles in water-based nanofluids using molecular dynamics simulation.
In our study, we have discussed about the rheological and diffusive properties of nanoparticle in aqueous medium since the nanoparticles hold greater effect on altering the rheological regime of the solution. Moreover, we have analytically solved the full interactive system integrals to evaluate the viscosity through the Green–Kubo formula and compared it with the results of the experimental approximation. Furthermore, the viscosity of the CeO2 nanoparticles in water system has not yet been known, since there is less research with respect to the viscosity calculation of this nanoparticle. There are some research available regarding viscosity analysis of CuO nanoparticles [13, 14] and Al2O3 nanoparticles dispersed in water [15, 16]. Thereby, the necessity of this research can be understood from our previous statement for other metal oxide nanoparticles application and experimental studies, since in this research experimental results trends have been compared with the simulative achieved results.
However, this study has been adopted from the review of molecular dynamics approach for nanoparticle dispersion simulation as proposed by Loya et al. [17].
Methodology
CeO2 nanoparticles of 10–40 nm diameters and 99.9 % purity (Shandong Yitong, China) were first dispersed in water by ultrasonication device for 3 min at 200 watts using Hielschers US200 ultrasonicator. Later, the nanofluids viscosity was measured using a rheometer, i.e. TA instrument 1500Ex. The measurement was taken from 293 to 323 K.
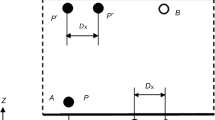
Moreover, simulations were performed on CeO2 nanoparticles dispersed in TIP3P water using LAMMPS [18]. A CeO2 nanoparticle was formed using Material studio from Accelrys Ltd UK. Later, both nanoparticle and water were introduced in one system. Charges on the atoms were assigned using COMPASS force field [19]. Moreover, two pair potentials, i.e. DPD and SPH [20] in hybrid style were used to execute the hydrodynamics and Brownian motion in the system. There were 40 CeO2 nanoparticles of 1 nm in size, within 8000 TIP3P water molecular system as shown in Fig. 1. The first system consisted of around 10 % volume fractions of particles. The second system consisted of 4 CeO2 nanoparticles of 1 nm that hold approximately 1 % of volume fractions. The system was equilibrated for three different temperature settings between 293 and 323 K.
Results
The viscosity of CeO2 nanoparticle dispersion was analysed by a TA 1500Ex instrument. The experimental viscosity trends were gathered and compared with the obtained simulation results. As the nanoparticles were similar in their volumetric concentrations, remarkable trends were achieved and were compared. The data from the simulation were quantified for their accuracy by stress autocorrelation functions (SACF).
The time taken to simulate depends on the number of atoms in the system and the statistical analysis required to be performed. The correct selection of the time step is necessary for computation. Since this correlates with the decaying of the SACF, it is necessary for the SACF to decay monotonically to analyse the real statistical observation of average viscosity. Therefore, the results of the viscosity achieved through simulation are convincible till the SACF decay monotonically to zero as shown in Fig. 6, which was the case found with our simulation results as shown in Fig. 2.
Furthermore, the analysis also used radial pair distribution functions. This function mathematically helps to determine the atomic pair distance between two consecutive atoms. This function is approached for agglomeration and dispersibility analysis.
Viscosity
As noted in Eq. 1, the simulation viscosity calculation carried out in this study was done using the Green–Kubo method. This method performs non-equilibrium molecular dynamics. The results of the viscosity measured by experiment and simulation are presented in Fig. 2.
Non-equilibrium methods have become increasingly popular for calculating the viscosity of liquids. The application of non-equilibrium methods is often simplified by the concept of pressure fluctuation-based equilibrium methods as shown in Eq. 1, such as those using the Green–Kubo method.
The results of the viscosity of water with nanoparticles were in good agreement with the results obtained from simulations, but the correlation function took a lot of time to converge, which is shown in Fig. 6. The trends of the simulation viscosity result were concurrent to the experimental achieved values; however, they were within 5 % of discrepancies.
Later, the stress tensor is used to validate the accuracy of achieved viscosity reading by integrating the stress tensor with respect to time correlation as presented in “Stress autocorrelation function”.
Mean square displacement
Mean square displacement was used to calculate the diffusion coefficient of the system by taking the slope of the line achieved from the diffusion trajectories of the simulation [21]. The diffusion coefficient was also calculated to analyse the system aggregation and viscous regime. However, the diffusion in the CeO–water system is due to the layering of nanoparticles with water molecules and nanoparticle–nanoparticle layer forming a stabilized system. Hydrodynamic layering also affects the diffusion, especially in our case, as we are using water, the effect of hydrodynamic layering cannot be neglected. The TIP3P water self-diffusion coefficient is roughly 0.5–0.7E−9 m2/s as investigated [22]. This shows that the results achieved in this study are realistic [23, 24]. The diffusion coefficients were calculated by Eq. 2 and are presented in Fig. 3.
Discussion
As already discussed in the results section, in the nanoparticle–water system, important factors that contribute towards the particle–particle interactions and changing the dispersibility of the system are diffusion coefficient and viscosity. Later, the viscosity achieved from the simulation can be further validated using the stress autocorrelation function; radial distribution function has been discussed to understand the aggregation nature of the CeO2 nanoparticle system.
Radial distribution function
Radial distribution function, also known as the pair distribution function, is the essential link between macroscopic thermodynamic properties and intermolecular interactions of fluids and fluid mixtures. RDF theories were effective in describing the behaviour of simple liquids and liquid mixtures. Ce atoms in water system show less aggregation as seen from Fig. 4. At 0.3–0.4 nm a strong peak is observed due to the Ce–Ce interaction within the nanoparticle bond, whereas the peaks after 2 nm shows lower intensities; these peaks relate to the distance between Ce atom of one nanoparticle to another nanoparticle.
Further, RDF is used for understanding the self-assembly (i.e. aggregation) of nanoparticles, that can be concerned as a fundamental question and this is also dependent on atom to atom shearing stress, i.e. the viscosity of the system. In this way, the interactions involved in controlling the self-assembly and aggregation mechanism can be understood. However, the experimental determination of these forces still remains a challenging task. On the other hand, theoretical methodologies such as radial distribution function in MD can be used to help understand the atomic interactions.
Density
Later, the density was analysed through MD simulation as shown in Fig. 5 and compared with the experimental and theoretical density. The theoretical density was calculated using Eq. 3. Whereas experimental density was found to be around 1.02 kg/m3, the theoretical density was calculated to be around 1.06 kg/m3. The difference between the theoretical and MD density is low. This shows (Fig. 6) that the system was properly configured.
Stress autocorrelation function
Validation of the measured viscosity is carried out through stress tensors. The stress tensor equation Eq. 4 [25] has three components for controlling the stress in a particular direction. This formula can be used with molecular dynamics simulations. However, alternative formulations based on particle displacement require translational invariance, an assumption violated in MD simulations utilizing periodic boundary conditions. The summation within the angle brackets in Eq. 4 implies averaging of a ‘adequately large’ number of samples [26]. The specified term P xy in Eq. 4 is the tensor for x–y direction, whereas there are other independent terms of the shear viscosity that are used as off-diagonal components of the stress. These terms are P xz and P yz . To implement Eq. 4, the optimal way is to use specific quantities. These quantities include the duration of the MD simulation, Δt, and the number of particles, N.
The monotonic decay of stress autocorrelation function for the CeO2–water nanoparticle system presented different time steps for various temperatures, i.e. for 303 K, the system decayed in 0.125 fs, whereas for 313 K it decayed in 0.135 fs and for 323 K decayed in 0.15 fs. Further, the SACF showed that the decaying function relies on the viscosity validation. The viscosity validation is achieved when the SACF reaches a monotonic decaying trend.
Comparison
The outcomes of this study are comparable to the study of CuO nanoparticles diffusion in water system as investigated by Loya et al. [27]. It is found that the metal oxide nanoparticles give similar diffusion rate when diffused in water. As shown in Table 1, in both cases, water diffusion coefficient is lower than the CuO–water system and CeO2–water system since nanoparticle enhances the random motion of atoms in the system.
Furthermore, the viscosity analysed using MD is within 5 % of discrepancies to experimental obtained values, which can be seen from Fig. 2. Nevertheless, these discrepancies are caused due to the velocity random seeding of the temperature fluctuation. During the system equilibration process, the pressure fluctuation can also be a part of causing discrepancies between MD and experimental values.
The standard error for diffusion of the CeO2–water system was calculated to estimate the accuracy for the results obtained from simulation as shown in Table 2. The standard error was calculated by dividing the simulation time steps into 10 blocks. Later at each block, diffusion coefficient was calculated. It was found that the results of average diffusion were around 1.5E−8 m2/s for 10 blocks and the overall standard error was found to be about 30–33 %.
Conclusion
In the present work, MD method was used to simulate nanoparticle dispersion at an atomistic level in aqueous solution. The cerium oxide (CeO2) nanoparticles were chosen as a model because this metal oxide nanoparticle has several important applications, such as fuel cell electrolyte, catalyst, polishing materials, insulators, gas sensor, and UV blockers.
However, the study demonstrated high concurrent trends in contrast to the experimental study with discrepancies of 5 % between both studies. Although we have used a small size and small number of nanoparticles, it shows advancement in understanding nanoparticles aggregation and dispersibility at atomic level. Moreover, this study is necessary for the development and achieving initial parameters for further implementation on future simulations for various nanoparticles–fluid systems.
Abbreviations
- CuO:
-
Copper Oxide
- CeO2:
-
Cerium Oxide
- Al2O3:
-
Aluminum oxide
- SiO2:
-
Silicon dioxide
- CeO–water system:
-
Cerium oxide–water system
- DPD:
-
Discrete particle dynamics
- MD:
-
Molecular dynamics
- SPH:
-
Smoothed Particle hydrodynamics
- UV:
-
Ultraviolet
- °K:
-
Degree Kelvin
- mPa.s:
-
Millipascal second
- COMPASS:
-
Condensed-phase Optimized Molecular Potential for Atomistic Simulation studies
- TA instruments:
-
Thermal analysis instruments
- r 0 :
-
Initial position of atom
- r i :
-
Distance travelled by atom from initial position
- P xy :
-
Pressure tensor in x–y direction
- V :
-
Volume
- K B :
-
Boltzmann constant
- T :
-
Temperature
- LAMMPS:
-
Large-scale atomic/molecular massively parallel simulator
- TIP3P:
-
Transferable intermolecular potential 3P
- η :
-
Viscosity
- D :
-
Diffusion coefficient
- t :
-
Time period
- C xy :
-
Stress component in x–y direction
- ϕ :
-
Concentration of particles
- ρ nf :
-
Density of nanofluid
- ρ p :
-
Density of nanoparticles
- ρ f :
-
Density of fluid
References
Zhao, C., Chen, Y.K., Ren, G.: A study of tribological properties of water-based ceria nanofluids. Tribol. Trans. 56(2), 275–283 (2013). doi:10.1080/10402004.2012.748948
Tiwari, A.K., Ghosh, P., Sarkar, J.: Performance comparison of the plate heat exchanger using different nanofluids. Experimental Thermal and Fluid Science 49(0), 141–151 (2013). doi:10.1016/j.expthermflusci.2013.04.012
Ngoc Nhiem, D., Minh Dai, L., Quang Khuyen, N., Byung Sun, K.: UV absorption by cerium oxide nanoparticles/epoxy composite thin films. Adv. Nat. Sci.: Nanosci. Nanotechnol. 2(4), 045013 (2011)
Hernández Battez, A., González, R., Viesca, J.L., Fernández, J.E., Díaz Fernández, J.M., Machado, A., Chou, R., Riba, J.: CuO, ZrO2 and ZnO nanoparticles as antiwear additive in oil lubricants. Wear 265(3–4), 422–428 (2008). doi:10.1016/j.wear.2007.11.013
Shriram S. Sonawane, R.S.K., Kailas L. Wasewar and Ajit P.: Rathod dispersions of CuO nanoparticles in paraffin prepared by Ultrasonication: a potential coolant 3rd International Conference on Biology, Environment and Chemistry IPCBEE vol.46 (2012) © (2012)IACSIT Press, Singapore (2012)
Jesumathy, S., Udayakumar, M., Suresh, S.: Experimental study of enhanced heat transfer by addition of CuO nanoparticle. Heat Mass Transfer 48(6), 965–978 (2012). doi:10.1007/s00231-011-0945-y
Zhao, C., Chen, Y.K., Jiao, Y., Loya, A., Ren, G.G.: The preparation and tribological properties of surface modified zinc borate ultrafine powder as a lubricant additive in liquid paraffin. Tribology International 70(0), 155–164 (2014). doi:10.1016/j.triboint.2013.10.007
Conesa, J.C.: Computer modeling of surfaces and defects on cerium dioxide. Surf. Sci. 339(3), 337–352 (1995). doi:10.1016/0039-6028(95)00595-1
Milanova, D., Kumar, R., Kuchibhatla, S., Seal, S.: Heat transfer behavior of oxide nanoparticles in pool boiling experiment. In: 2006. ASME
Gotte, A., Hermansson, K., Baudin, M.: Molecular dynamics simulations of reduced CeO < sub > 2 </sub > : bulk and surfaces. Surf. Sci. 552(1), 273–280 (2004)
Gotte, A., Spångberg, D., Hermansson, K., Baudin, M.: Molecular dynamics study of oxygen self-diffusion in reduced CeO2. Solid State Ionics 178(25), 1421–1427 (2007)
Herschend, B., Baudin, M., Hermansson, K.: Oxygen vacancy formation for transient structures on the CeO (110) surface at 300 and 750 K. J. Chem. Phys. 126, 234706 (2007)
Karimi, H., Yousefi, F., Rahimi, M.: Correlation of viscosity in nanofluids using genetic algorithm-neural network (GA-NN). Heat Mass Transfer 47(11), 1417–1425 (2011). doi:10.1007/s00231-011-0802-z
Nguyen, C.T., Desgranges, F., Roy, G., Galanis, N., Maré, T., Boucher, S., Angue Mintsa, H.: Temperature and particle-size dependent viscosity data for water-based nanofluids––Hysteresis phenomenon. Intern. J. Heat Fluid Flow 28(6), 1492–1506 (2007). doi:10.1016/j.ijheatfluidflow.2007.02.004
Duan, F., Kwek, D., Crivoi, A.: Viscosity affected by nanoparticle aggregation in Al2O3-water nanofluids. Nanoscale Res. Lett. 6(1), 248 (2011)
Nguyen, C.T., Desgranges, F., Galanis, N., Roy, G., Maré, T., Boucher, S., Angue Mintsa, H.: Viscosity data for Al2O3–water nanofluid—hysteresis: is heat transfer enhancement using nanofluids reliable? Intern. J. Thermal Sci. 47(2), 103–111 (2008). doi:10.1016/j.ijthermalsci.2007.01.033
Loya, A., Stair, J.L., Ren, G.: The Approach of using Molecular Dynamics for Nanofluid Simulations. Intern. J. Eng. Res. Technol 3(5), 1236–1247 (2014). http://www.ijert.org/view.php?id=9756&title=the-approach-of-using-molecular-dynamics-for-nanofluid-simulations
Plimpton, S.: Fast parallel algorithms for short-range molecular dynamics. J. Comp. Phys. 117, 1–19 (1995)
Sun, H.: COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 102(38), 7338–7364 (1998). doi:10.1021/jp980939v
Gingold, R.A., Monaghan, J.J.: Smoothed particle hydrodynamics: theory and application to non-spherical stars. Mon. Not. R. Astron. Soc. 181, 375–389 (1977)
Fan, H.B., Chan, E.K.L., Wong, C.K.Y., Yuen, M.M.F.: Moisture diffusion study in electronic packaging using molecular dynamic simulation. In: Electronic Components and Technology Conference, 2006. Proceedings. 56th, 0-0 0 2006, 4
Mark, P., Nilsson, L.: Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 105(43), 9954–9960 (2001). doi:10.1021/jp003020w
Allen, M.P., Tildesley, D.J.: Computer simulation of liquids. Oxford University Press, (1989)
Alder, B.J., Gass, D.M., Wainwright, T.E.: Studies in Molecular Dynamics. VIII. The Transport Coefficients for a Hard-Sphere Fluid. J. Chem. Phys. 53(10), 3813–3826 (1970)
Kubo, R.: Statistical-mechanical theory of irreversible processes. I. general theory and simple applications to magnetic and conduction problems. J. Phys. Soc. Jpn. 12(Copyright (C) 1957 The Physical Society of Japan), 570
Rapaport, D.C.: The art of molecular dynamics simulation. Cambridge University Press, (2004)
Loya, A., Stair, J.L., Ren, G.: The study of simulating metaloxide nanoparticles in aqueous fluid. Intern. J. Eng. Res Technol 3(4), 1954–1960 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Loya, A., Stair, J.L. & Ren, G. Simulation and experimental study of rheological properties of CeO2–water nanofluid. Int Nano Lett 5, 1–7 (2015). https://doi.org/10.1007/s40089-014-0129-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-014-0129-0