Abstract
l-Carnitine plays a key role in the regulation of energy metabolism and growth in fish. The aim of this study was to determine the effects of dietary l-carnitine levels on carnitine homeostasis and energy metabolism in diploid and triploid trout (Oncorhynchus mykiss). Diploid and triploid trout (29.5 ± 0.6 and 31.8 ± 0.5 g, respectively) were fed with three diets supplemented with 15, 200 or 530 mg l-carnitine/kg, respectively, for 56 days. Compared to 15 mg l-carnitine, total carnitine content increased in the liver by 182% in diploid and by 154% in triploid trout fed 530 mg l-carnitine. In muscle, total carnitine content increased by 60 and 73% in diploid and by 34 and 75% in triploid with the increase in dietary l-carnitine levels. l-Carnitine caused no significant change in the plasma concentrations of metabolites like proteins (ammonia, urea and protein), lipid (triglycerides), carbohydrate (glucose, lactate) and enzyme activities (lipase, lactate, alanine transaminase, lactic acid dehydrogenase). Triploid trout had significantly lower plasma ammonia (P = 0.003), lipase (P = 0.005) and triiodothyronine (T3; P = 0.003) levels than diploid trout. In conclusion, ploidy significantly affected the energy metabolism in rainbow trout, dietary l-carnitine levels altered the l-carnitine homeostasis, but not influence nutritional metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past three decades, aquaculture production has increased steadily, an average of 9% year, reaching a staggering 60 million tones (excluding aquatic plants and non-food products) in 2010 (FAO 2012). Fish meal and fish oil derived from wild catches are dominant ingredients in aqua feed. It is estimated, at the present annual growth rates, that fishmeal supplies will be entirely consumed by the aquaculture sector by 2025. Plant protein ingredients have increasingly been used in diets of aquaculture species (Glencross Booth and Allan 2007), including carnivore marine species (Oliva-Teles and Gonçalves 2001; Colburn et al. 2012), is crucial the reduction of aquaculture farming dependent on fish meal. The replacement of fish meal may trigger, however, specific deficiency symptoms, including l-carnitine deficiency. Fishmeal may contain 10–20 times more l-carnitine than plant-based feeds (Ozório et al. 2001).
l-Carnitine, a multi-physiological and bioactive vitamin-like nutrient, plays an important role in cellular nutritional metabolic status, including the regulation of fish energy metabolism and growth (Harpaz 2005; Mohseni et al. 2008). Fish fed high-fat diets supplemented with 600 mg l-carnitine showed a reduction in plasma glucose and lactate dehydrogenase (LDH) (Ozório et al. 2003) suggesting an inhibitory effect on pyruvate dehydrogenase complex (PDH), the key rate-limiting step in carbohydrate oxidation (Randle 1998; Rasmussen and Wolfe 1999) or enhancement of glucose flux toward lipogenesis. A decrease in LDH levels may also indicate an increase protection of l-carnitine against oxidative stress (Ronca et al. 1992).
According to Rasmussen and Wolfe (1999) growth is impaired when a dietary l-carnitine level is limited. In fish, an increase in energy demand is followed by the increase in lipid metabolism and body l-carnitine turnover (Ozório et al. 2001). Carnitine reserves are mainly replenished by the diet since carnitine biosynthesis is not sufficient, especially in very young animals (Harpaz 2005). Ozório et al. (2008) observed an improvement in survival, cell viability and ventilation frequency of turbot Psetta maxima L. juveniles fed l-carnitine supplements following acute exposure to toxic ammonia levels and to pathogen agent (S. parauberis). In case of fishmeal replacement, body l-carnitine reserves may become limited, thus dietary supplementation is required.
In salmonid production, sterile triploids have been increasingly used. After the onset of sexual maturation, triploid fish often grow faster and have better flesh quality than their diploid counterparts. In addition, triploid fish may display greater hypertrophic growth and reduced energy costs for gamete production (Thorgaard 1986; Qin et al. 1998; Oppedal et al. 2003; Maxime 2008). Moreover, its use in aquaculture may minimize the risks of genetic losses from wild populations (O’Flynn et al. 1997; Benfey 1999; Atkins and Benfey 2008).
The comparative results between diploid and triploid fish are often contradictory (Ihssen et al. 1990; Galbreath et al. 1994; Li et al. 2017). The general consensus is that the survival and growth are substantially lower in the early life stages in triploids compared to diploids (Thorgaard 1986; Sutterlin et al. 1987; Malison et al. 1993; Withler et al. 1995). In addition, triploids appear to be less resilient and less tolerant to poor water quality than their diploid counterpart (Benfey 1999; Benfey and Biron 2000; Altimiras et al. 2002; Segato et al. 2006; Atkins and Benfey 2008; Maxime 2008).
The aim of this study was to assess the effects of dietary l-carnitine levels on energy metabolism in diploid and triploid rainbow trout (Oncorhynchus mykiss).
Materials and methods
Fish and husbandry conditions
The current study is an extension of a published study (Ozório et al. 2012) in which the results of growth performance and body composition has been presented. The growth trial was carried out in accordance with European Directive 86/609/UE by a certified researchers under National and International animal welfare laws, according to the FELASA requirements (level C).
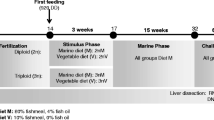
Two groups of 5-month-old rainbow trout were used, one of all-female diploid trout (initial weight 29.5 ± 0.6 g) and another of triploid trout (initial weight 31.8 ± 0.5 g). Fish were outbred siblings and had identical nutritional and husbandry history and were kept in standard culture conditions at a density of 15–20 kg/m3. After an acclimatization period of 2 weeks, 612 rainbow trout were individually weighed and randomly distributed in 18 fiberglass tanks of 50 L. The water supply system consisted of a closed circuit (TMC-5000, TMC Iberia, Lisbon, Portugal), which included mechanical and biological filtration, a skimmer, an ultraviolet sterilization unit and a refrigerator. The water quality parameters such as temperature (16.9 ± 0.5 °C), pH (7.3 ± 0.1) dissolved oxygen (8.0 ± 0.4 mg/L), ammonia (0.6 ± 0.8 mg/L) and nitrite (1.2 ± 0.5 mg/L) were checked on a daily basis. Photoperiod (12 h L/12 h D) and light intensity (200 Lux) was kept constant.
Diets, sampling and experimental design
The experimental diets were formulated to contain three dietary l-carnitine levels (15, 200 or 530 mg/kg diet) (Table 1) and fed in triplicate to diploid and triploid trout groups for a period of 56 days. All diets were iso-proteic (44% crude protein) and isocaloric (20 MJ/kg) and had identical formulation, except for l-carnitine content. Since the concentration of 200 mg l-carnitine per kilogram diet is the generally obtained in commercial aquafeed, the low l-carnitine level (15 mg/kg diet) was possible using several plant protein ingredients know to have very low levels or deficient in l-carnitine in replacement of animal ingredients, rich in l-carnitine. The 530 mg l-carnitine concentration was selected based on previous studies, which have showed that l-carnitine improved performance until a dietary level of 600 mg/kg, with best performance around 500 mg l-carnitine/kg diet (Ozório 2001; Ozório et al. 2001, 2012). Fish were fed twice daily (10:00 and 15:00 h), to apparent satiation.
At the end of the trial all fish were starved for 24 h prior to sampling. Before sampling, fish were euthanized by an overdose bath of 0.4 mL ethylene-glycol–monophenol–ether/L water (Merck, Darmstadt, Germany). A total of 12 fish per group were sampled for muscle, liver and blood plasma analyses. The samples collected were stored at − 80 °C, until further processing.
Growth performance
The results of growth performance parameters are presented solely in the text of the Result section. For detailed information about the growth performance, see Ozório et al. (2012). The voluntary feed intake (VFI), feed conversion ratio (FCR) and growth rate (daily growth index, DGI) was calculated as follows:
Carnitine analyses
Diets, liver, and muscle tissues were analyzed for the free- and acyl l-carnitine content. All samples, prior to l-carnitine analysis, were deproteinated with perchloric acid, subsequently neutralized with potassium hydroxide, centrifuged and the supernatant collected.
l-Carnitine was extracted by stepwise heating, ultrasonic treatment or extraction with various detergents. Additionally, alkaline hydrolysis was performed for the determination of l-carnitine esters. The l-carnitine determination in diets and tissues was performed by radiometric detection of free and l-carnitine esters. The assay is based on the reaction of free l-carnitine with acetyl-CoA catalyzed by carnitine acetyltransferase with the production of acetyl carnitine and coenzyme (Christiansen and Bremer 1978).
Plasma analyses
Blood was collected by caudal puncture with lithium-heparin coated syringes. Blood was immediately centrifuged (10 min at 1500 g at 4 °C) and plasma stored at − 80 °C until subsequent analysis. All plasma tests were performed in triplicate. Plasma cortisol (ng/mL) was quantified by Radioimmunoassay (RIA) technique, using commercial kits (Cortisol Coat-A-Count, DPC, Los Angeles, CA, USA) validated for salmonids.
The total ammonia concentration in plasma was determined using the method described by Bergmeyer and Beutler (1983) and plasma osmotic pressure (mOsm) was determined with an osmometer (model 15—Löser Messtechnik, Berlin, Germany). Plasma glucose concentrations were measured by the glucose oxidase method using a Beckman glucose analyzer 2 (Beckman Instruments, Brea, CA, USA).
Urea was quantified colourimetrically with the Glutamate dehydrogenase (GLDH) enzymatic method. Lipase was quantified colourimetrically with an enzymatic method using 1,2-0-dilauryl-rac-glycero-3-glutaric acid-(6′-methyl resorufin)-ester as subtracting. Alanine aminotransferase (ALT) was quantified by an enzymatic method in accordance with IFCC. Lactate dehydrogenase (LDH) was quantified by an enzymatic method in accordance with IFCC. Lactate was quantified colourimetrically with the lactate oxidase/peroxidase enzymatic method and protein was quantified colourimetrically with the biuret method. Triglyceride was quantified colourimetrically with the glycerol phosphate oxidase/peroxidase enzymatic method. All the methods were executed in a Roche Cobas Integra 800 Chemistry Analyser (Roche® Sistemas de Diagnóstico, Amadora, Portugal), with Roche reagents. Triiodothyronine (T3) and thyroxine (T4) were quantified by electrochemiluminescence immunoassays in a Roche Cobas e 411 Analyser (Roche® Sistemas de Diagnóstico, Amadora, Portugal), with Roche reagents.
Statistical analyses
All data were tested for normality using Kolmogorov–Smirnov and Levene tests and analyzed using two-way analyses of variance (ANOVA). The model included the effect of ploidy (two levels) and dietary l-carnitine (three levels) and their interaction. Data were analyzed using the General Linear Model procedure (SAS Institute Inc., Cary, NC, USA). The results (mean ± standard deviation) are reported as significant at P < 0.05.
Results
Voluntary feed intake (VFI: 1.77–1.99% BW/day) was significantly affected by ploidy and l-carnitine levels (P < 0.001). Feed conversion ratio (FCR: 0.95–1.07 g/g) was significantly affect by dietary l-carnitine levels (P < 0.05). Growth rate (2.52–2.65% BW/day) was not affected by diet or ploidy.
The concentration of l-carnitine fractions (free and acyl l-carnitine) significantly increased in muscle and liver (Fig. 1) with the dietary l-carnitine intake (Fig. 2). Muscle acyl l-carnitine content of fish fed 15, 200 and 530 mg l-carnitine was significantly higher (P = 0.003) in triploid (19.4, 26.6 and 33.6 mg/kg) than in diploid trout (15.6, 25.3 and 26.5 mg/kg). Free carnitine content was significantly higher than acyl l-carnitine content in both muscle and liver tissues.
Ploidy and l-carnitine supplementation did not affect plasma glucose, urea, protein, and triglyceride levels (Table 2). Diploid trout fed 200 and 530 mg l-carnitine had the highest (513 ± 67.78 U/L) and the lowest LDH levels (404.0 ± 10.0 U/L), respectively. Triploid fish had significantly lower plasma ammonia (P = 0.003), lipase (P = 0.005) and triiodothyronine (T3, P = 0.003) levels than diploid trout. Trout fed 200 mg l-carnitine had the lowest ammonia (8.89 ± 0.64 µg/mL) and T3 (1.84 ± 0.31 ng/mL) levels, while trout fed 530 mg l-carnitine had the lowest lipase level (2.80 ± 0.51 U/L). Plasma cortisol ranged from 4.8 ± 1.6 to 7.8 ± 3.3 ng/mL and was not significantly affected by the different treatments. Plasma osmolality was significantly affected by ploidy (P = 0.008), with triploid trout having lower osmolality (298.2 ± 7.1 mOsm) than diploid trout (303.5 ± 3.3 mOsm).
Discussion
l-Carnitine supplementation improved growth performance when fed to fish larvae and early juvenile fish species (Santulli and D’Amello 1986; Torreele et al. 1993; Chatzifotis et al. 1995; Keshavanath and Renuka 1998). In studies with larger animals, the positive effects of l-carnitine become less evident, as confirmed by Chatzifotis et al. (1996). In the current study using juveniles, with exception of FCR, l-carnitine supplementation did not improve growth performance. The ambiguity among many dose–response studies on l-carnitine may be caused by different l-carnitine concentrations, the husbandry conditions or the duration of the dietary treatment (Ozório et al. 2012).
The carnitine pool in animals is maintained by a combination of absorption of carnitine from supplemental sources, a modest rate of biosynthesis, and an efficient reabsorption of carnitine (Rebouche and Seim 1998). The body distribution of carnitine is determined by a series of systems that transport carnitine into cells against a concentration gradient. The liver plays a unique role in the whole-body carnitine homeostasis. The liver transport system has a higher affinity for butyrobetaine (Km ≈ 0.5 mM) (Christiansen and Bremer 1978; Kerner and Hoppel 1998) than for carnitine (Km ≈ 5.0 mM), this high affinity for butyrobetaine favors its transport into the liver and the efflux system acyl l-carnitine over l-carnitine (Hokland 1988).
Free and acyl l-carnitine content increased with the dietary l-carnitine supplementation, whereas higher levels were observed in muscle than in liver, indicating a direct relationship between the dietary and body l-carnitine. The muscle may store a large amount of l-carnitine against a concentration gradient by an active transport process, which could be to ensure an immediate and efficient energy for the muscle during exercise and recovery. Ozório et al. (2001) working with African catfish Clarias gariepinus observed that the muscle l-carnitine concentration was about 12 times higher than plasma l-carnitine. The l-carnitine concentration gradient was even more pronounced between muscle and liver tissues (Ozório et al. 2001).
The ability to take up and retain dietary carnitine was observed in other species, such as Atlantic salmon Salmo salar L. (Ji et al. 1996), red seabream Pagrus major (Chatzifotis and Takeuchi 1997), rabbits (Bell et al. 1992), piglets (Böhles et al. 1984), rats (Tsai et al. 1974), among other species (Li et al. 2017).
The increase in the acyl l-carnitine fraction in fish tissues may indicate an increase in lipid catabolism and that the diet quality and quantity may affect the body l-carnitine level. These results are consistent with the study described by Ozório et al. (2001), which demonstrated that the concentration of l-carnitine in muscle differs between fish fed with low and high l-carnitine levels. In fact, muscle l-carnitine levels increased moderately when African catfish were fed between 40 and 600 mg l-carnitine. Thereafter, a sharp increase in muscle l-carnitine level was observed. The latter suggests that l-carnitine requirement in catfish, under a normal physiological condition, would be below 600 mg/kg diet.
In the current study, the ploidy affected the acyl l-carnitine content in muscle, with higher values observed in triploid fish. This could indicate that triploid trout have higher l-carnitine requirement than diploid fish. In fact, triploid fish have a lower respiratory efficiency and are more susceptible to stress than diploid fish. Triploid fish have fewer red blood cells which probably affect the exchange of oxygen and decrease the ability to transport oxygen in the blood (Benfey 1999; Benfey and Biron 2000; Tiwary and Ray 2004) and, therefore, spend more energy to maintain internal balance than their diploid counterpart, possibly mobilizing more body l-carnitine for energy production. Plasma lipase was higher in diploid fish and is in agreement with the slight increase in plasma triglyceride. Lipase is the rate-limiting enzyme for the hydrolysis of triglyceride in plasma (Albalat et al. 2006).
Plasma cortisol levels were similar to the basal levels observed for this species (< 20 ng/mL) (Taylor et al. 2007), with no effect of diet and ploidy. In the current study, triploid trout had lower plasma ammonia concentrations, which may reflect on a higher capacity to excrete ammonia than diploid trout. Considering that ammonia excretion rates are linked to acid–base regulation (Kieffer and Tufts 1996), triploid rainbow trout may have a greater clearance capacity of metabolic protons. This would allow for faster recovery from acidosis and energy (ATP) depletion after exercise, which is often the case during handling stress. A faster recovery of the blood acid–base status may also reflect a faster recovery of ion and osmoregulatory imbalance, which was also shown by the significant differences in plasma osmolality between triploid and diploid trout. Plasma triiodothyronine hormone (T3) was significantly higher in diploid than triploid trout, as well as the feed intake (VFI). Feed intake, quality and quantity, is known to influence thyroid hormone levels in rainbow trout (Leatherland et al. 1977; Himick et al. 1991). Thyroid hormones interact closely with the energy metabolism, with T3 playing a role in the regulation of growth and nutrient partitioning in teleosts (Peter and Marchant 1995; Korytko and Cuttler 1997).
In the current study triploid trout had lower plasma LDH level. As mentioned earlier, triploid fish have lower oxygen transport ability, which may explain their lower activity when compared to diploid trout. According to Sullivan and Somero (1980), fish with different locomotors activity would have different LDH activity to compensate for the amount of lactate produced. Wardle (1978) reported lower lactate ion concentration in flatfish than in rainbow trout.
Conclusion
Although a multitude of questions concerning the regulation of dietary carnitine requirements remain to be elucidated, our study suggested that ploidy significantly affected the energy metabolism, whereas dietary l-carnitine levels altered the carnitine homeostasis, but did not influence energy metabolism in diploid and triploid rainbow trout.
Abbreviations
- ABW:
-
Average body weight
- ALT:
-
Alanine aminotransferase
- ATP:
-
Adenosine triphosphate
- BW:
-
Body weight
- DGI:
-
Daily growth index
- FBW:
-
Final body weight
- FCR:
-
Feed conversion ratio
- GLDH:
-
Glutamate dehydrogenase
- IBW:
-
Initial body weight
- IFCC:
-
International Federation of Clinical Chemistry and Laboratory Medicine
- LDH:
-
Lactate dehydrogenase
- VFI:
-
Voluntary feed intake
References
Albalat A, Sánchez-Gurmaches J, Gutiérrez J, Navarro I (2006) Regulation of lipoprotein lipase activity in rainbow trout (Oncorhynchus mykiss) tissues. Gen Comp Endocrinol 146:226–235
Altimiras J, Axelsson M, Claireaux G, Lefrançois C, Mercier C, Farrell AP (2002) Cardiorespiratory status of triploid brown trout during swimming at two acclimation temperatures. J Fish Biol 60:102–116
Atkins ME, Benfey TJ (2008) Effect of acclimation temperature on routine metabolic rate in triploid salmonids. Comp Biochem Physiol A Mol Integr Physiol 149:157–161
Bell FP, Vidmar TJ, Raymond TL (1992) l-Carnitine administration and withdrawal affect plasma and hepatic carnitine concentrations, plasma lipid and lipoprotein composition, and in vitro hepatic lipogenesis from labeled mevalonate and oleate in normal rabbits. J Nutr 122:959–966
Benfey TJ (1999) The physiology and behavior of triploid fishes. Rev Fish Sci 7:39–67
Benfey T, Biron M (2000) Acute stress response in triploid rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis). Aquaculture 184:167–176
Bergmeyer HU, Beutler HO (1983) Ammonia. Methods Enzymat Anal 8:454–461
Böhles H, Michalk D, Brandl U, Fekl W, Börresen HC, Stehr K (1984) The effect of l-carnitine-supplemented total parenteral nutrition on tissue amino acid concentrations in piglets. J Nutr 114:671–676
Chatzifotis S, Takeuchi T (1997) Effect of supplemental carnitine on body weight loss, proximate and lipid compositions and carnitine content of red sea bream (Pagrus major) during starvation. Aquaculture 158:129–140
Chatzifotis S, Takeuchi T, Seikai T (1995) The effect of dietary l-carnitine on growth performance and lipid composition in red sea bream fingerlings. Fish Sci 61:1004–1008
Chatzifotis S, Takeuchi T, Seikai T (1996) The effect of dietary carnitine supplementation on growth of red sea bream (Pagrus major) fingerlings at two levels of dietary lysine. Aquaculture 147:235–248
Christiansen RZ, Bremer J (1978) Acetylation of tris(hydroxymethyl)aminomethane (tris) and tris derivatives by carnitine acetyltransferase. FEBS Lett 86:99–102
Colburn HR, Walker AB, Breton TS, Stilwell JM, Sidor IF, Gannam AL, Berlinsky DL (2012) Partial replacement of fishmeal with soybean meal and soy protein concentrate in diets of atlantic cod. N Am J Aquacult 74:330–337
FAO (2012) The state of world fisheries and aquaculture. FAO, Rome. www.fao.org/docrep/016/i2727e/i2727e00.htm
Galbreath PF, St Jean W, Anderson V, Thorgaard GH (1994) Freshwater performance of all-female diploid and triploid atlantic salmon. Aquaculture 128:41–49
Glencross BD, Booth M, Allan GL (2007) A feed is only as good as its ingredients—a review of ingredient evaluation strategies for aquaculture feeds. Aquacult Nutr 13:17–34
Harpaz S (2005) l-Carnitine and its attributed functions in fish culture and nutrition—a review. Aquaculture 249:3–21
Himick BA, Higgs DA, Eales JG (1991) The acute effects of alteration in the dietary concentrations of carbohydrate, protein, and lipid on plasma t4, t3, and glucose levels in rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol 82:451–458
Hokland BM (1988) Uptake, metabolism and release of carnitine and acylcarnitines in the perfused rat liver. Biochim Biophys Acta 961:234–241
Ihssen PE, McKay LR, McMillan I, Phillips RB (1990) Ploidy manipulation and gynogenesis in fishes: cytogenetic and fisheries applications. Trans Am Fish Soc 119:698–717
Ji H, Bradley TM, Tremblay GC (1996) Atlantic salmon (Salmo salar) fed l-carnitine exhibit altered intermediary metabolism and reduced tissue lipid, but no change in growth rate. J Nutr 126:1937–1950
Kerner J, Hoppel C (1998) Genetic disorders of carnitine metabolism and their nutritional management. Annu Rev Nutr 18:179–206
Keshavanath P, Renuka P (1998) Effect of dietary l-carnitine on growth and body composition of fingerling rohu, Labeo rohita (Hamilton). Aquacult Nutr 4:83–87
Kieffer JD, Tufts BL (1996) The influence of environmental temperature on the role of the rainbow trout gill in correcting the acid–base disturbance following exhaustive exercise. Physiol Zool 69:1301–1323
Korytko AI, Cuttler L (1997) Thyroid hormone and glucocorticoid regulation of pituitary growth hormone-releasing hormone receptor gene expression. J Endocrinol 152:R13–R17
Leatherland FJ, Cho CY, Slinger SJ (1977) Effects of diet, ambient temperature, and holding conditions on plasma thyroxine levels in rainbow trout Salmo gairdneri. J Fish Res Board Can 34:677–682
Li JM, Li LY, Qin X, Ning LJ, Lu DL, Li DL, Zhang ML, Wang X, Du ZY (2017) Systematic regulation of l-carnitine in nutritional metabolism in zebra-fish, Danio rerio. Sci Rep 7:40815
Malison JA, Procarione LS, Held JA, Kayes TB, Amundson CH (1993) The influence of triploidy and heat and hydrostatic pressure shocks on the growth and reproductive development of juvenile yellow perch (Perca jlavescens). Aquaculture 116:121–133
Maxime V (2008) The physiology of triploid fish: current knowledge and comparisons with diploid fish. Fish Fish 9:67–78
Mohseni M, Ozório ROA, Pourkazemi M, Bai SC (2008) Effects of dietary l-carnitine supplements on growth and body composition in beluga sturgeon (Huso huso) juveniles. J Appl Ichthyol 24:646–649
O’Flynn MF, McGeachy SA, Friars GW, Benfey TJ, Bailey JK (1997) Comparisons of cultured triploid and diploid atlantic salmon, Salmo salar L. ICES J Mar Sci 54:1160–1165
Oliva-Teles A, Gonçalves P (2001) Partial replacement of fishmeal by brewers yeast (Saccharomyces cerevisiae) in diets for sea bass (Dicentrarchus labrax) juveniles. Aquaculture 202:269–278
Oppedal F, Taranger GL, Hansen T (2003) Growth performance and sexual maturation in diploid and triploid atlantic salmon (Salmo salar L.) in seawater tanks exposed to continuous light or simulated natural photoperiod. Aquaculture 215:145–162
Ozório ROA (2001) Dietary l-carnitine and energy and lipid metabolism in African catfish (Clarias gariepinus) juveniles. 1 vols, p. 136. Wageningen: Ponsen and Looijen. (ISBN 90-5808-493-0), PhD Thesis
Ozório ROA, Uktoseja JL, Huisman EA, Verreth JA (2001) Changes in fatty acid concentrations in tissues of african catfish, Clarias gariepinus (Burchell), as a consequence of dietary carnitine, fat and lysine supplementation. Br J Nutr 86:623–636
Ozório ROA, Verreth JA, Aragão CR, Vermeulen CJ, Schrama JW, Verstegen MWA (2003) Dietary carnitine supplements increased lipid metabolism and decreased protein oxidation in african catfish (Clarias gariepinus) juveniles fed high fat levels. J Aquacult Trop 18:225–238
Ozório ROA, Gonçalves R, Dias S, Correia AD, Gonçalves J, Wilson J (2008) Protective effects of dietary l-carnitine on turbot, Psetta maxima, challenged with S. parauberis and sublethal ammonia concentration. In: Proceedings 13th international symposium on nutrition and feeding of fish, Florianópolis, Brazil. Aquacult Res 41(3): 168–168
Ozório ROA, Escorcio C, Bessa RJB, Ramos B, Gonçalves JFM (2012) Comparative effects of dietary l-carnitine supplementation on diploid and triploid rainbow trout (Oncorhynchus mykiss). Aquacult Nutr 18:189–201
Peter RE, Marchant TA (1995) The endocrinology of growth in carp and related species. Aquaculture 129:299–321
Qin JG, Fast AW, Ako H (1998) Growout performance of diploid and triploid chinese catfish Clarias fuscus. Aquaculture 166:247–258
Randle PJ (1998) Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Res Rev 14:263–283
Rasmussen BB, Wolfe RR (1999) Regulation of fatty acid oxidation in skeletal muscle. Annu Rev Nutr 19:463–484
Rebouche CJ, Seim H (1998) Carnitine metabolism and its regulation in microorganisms and mammals. Annu Rev Nutr 18:39–61
Ronca G, Ronca F, Yu G, Zucchi R, Bertelli A (1992) Protection of isolated perfused working rat heart from oxidative stress by exogenous l-propionyl carnitine. Drugs Exp Clin Res 18:475–480
Santulli A, D’Amello V (1986) The effects of carnitine on the growth of sea bass, Dicentrarchus labrax L. fry. J Fish Biol 28:81–86
Segato S, Bertotto D, Fasolato L, Francescon A, Barbaro A, Corato A (2006) Effects of triploidy on feed efficiency, morphometric indexes and chemical composition of shi drum, Umbrina cirrosa L. Aquacult Res 37:71–77
Sullivan KM, Somero GN (1980) Enzyme activities of fish skeletal muscle and brain as influenced by depth of occurrence and habits of feeding and locomotion. Mar Biol 60:91–99
Sutterlin AM, Holder J, Benfey TJ (1987) Early survival rates and subsequent morphological abnormalities in landlocked, anadromous and hybrid (landlocked × anadromous) diploid and triploid atlantic salmon. Aquaculture 64:157–164
Taylor JF, Needham MP, North BP, Morgan A, Thompson K, Migaud H (2007) The influence of ploidy on saltwater adaptation, acute stress response and immune function following seawater transfer in non-smolting rainbow trout. Gen Comp Endocrinol 152:314–325
Thorgaard GH (1986) Ploidy manipulation and performance. Aquaculture 57:57–64
Tiwary KB, Ray AK (2004) Alterations in air-sac and skeleton of triploid heteropneustes fossilis. J Fish Biol 64:268–272
Torreele E, van der Sluiszen A, Verreth J (1993) The effect of dietary l-carnitine on the growth performance in fingerlings of the african catfish (Clarias gariepinus) in relation to dietary lipid. Br J Nutr 69:289–299
Tsai CA, Romsos DR, Leveille GA (1974) Significance of dietary carnitine for growth and carnitine turnover in rats. J Nutr 104:782–792
Wardle CS (1978) Non-release of lactic acid from anaerobic swimming muscle of plaice Pleuronectes platessa L.: a stress reaction. J Exp Biol 77:141–155
Withler RE, Beacham TD, Solar II, Donaldson EM (1995) Freshwater growth, smolting, and marine survival and growth of diploid and triploid coho salmon (Oncorhynchus kisutch). Aquaculture 136:91–107
Acknowledgements
This research was partially supported by the European Regional Development Fund (ERDF) through the COMPETE—Operational Competitiveness Programme and national funds through FCT—Foundation for Science and Technology, under the project “PEst-C/MAR/LA0015/2011.
Author information
Authors and Affiliations
Contributions
All authors have contributed intellectually in this study. GJFM gave substantial contributions in the concept, design and interpretation of data, MJ was involved in revising the manuscript, HM and AS were involved in interpretation of data, drafting the manuscript and revising, OJC was more involved in acquisition of data, analysis and interpretation and OROA was involved in all phases of the study, supervising and giving intellectual feedback.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gonçalves, J.F.M., Hinzmann, M., Machado, J. et al. Oxidative effect of l-carnitine on energy metabolism in diploid and triploid rainbow trout (Oncorhynchus mykiss): impact on metabolites. Int Aquat Res 10, 133–143 (2018). https://doi.org/10.1007/s40071-018-0194-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40071-018-0194-5