Abstract
The gravel pits are typical features of Bratislava. By their origin they are related to the river Danube. The water quality is determinated by various indicators, especially hydrochemical and microbiological ones. In the gravel pits water was determined for increase of chloride concentration and drop of sulphate concentration. Significant indicator of faecal contamination is the presence of enterococci. Faecal enterococci (E.faecalis, E.faecium, E.durans, E.hirae) dominates in the river Danube, and only two non-faecal enterococcal species, E.casseliflavus and E. seriolicida were found in the water of gravel pits with other enterococcal very related taxa: Lactococcus lactis and Aeromonas spp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gravel pits filled with groundwater are typical features of Bratislava. They are witnesses of the gravel and sand exploitation of the Danube River alluvial deposits, and are important esthetic and urbanistic features used for public recreation, fishing and swimming. The gravel pits located in the Bratislava region had been mostly formed after World War II due to increasing demand for the gravel–sand material to rebuild the city.
Genetically, the water in the gravel pits is of a specific type. It originates from groundwater; the main source in the area of Bratislava is the water infiltrating from the Danube River. Along the left bank and close to Malé Karpaty Mts. The groundwater is also effected by flows from the mountain range. Chemical composition of the Danube water changes during the infiltration depending on hydrogeochemical activity of sediments, and on the degree of their contamination. When the water level is uncovered, it attains some of the properties characteristic for surface water.
Natural conditions
The monitored area is situated in the intermountain part of the Danube basin, called in Slovakia “Podunajská nížina” (Danubian Lowland). The basin consists of late tertiary sediments (marine and lacustrine sand, fine sand, clay, sandstone and shale) and Quaternary sediments. The Quaternary sub-base is made up of ruman, dak and pont sediments, mostly of grey up to grey-green weakly calcinated mica clays and dust with varying admixture of sand (Mucha et al. 2004). The Danube River sediments gravel and sand, deposited in the Danube alluvial and lacustrine conditions. The total depth of Quaternary sediments reaches in Bratislava area from a few metres to 450 m in central part of the Danubian Lowland. The main aquifer consisting of highly permeable gravels and sands with hydraulic conductivity coefficient usually from 0.0001 to 0.02 m/s (Mucha and Lisický 2006). The eastern part of the territory between the Danube and the Malý Dunaj is known under the name of Žitný ostrov.
Water from the Danube, therefore, infiltrates into the fan alluvial sediments during all water stage on the Danube river, and flows downward as groundwater through the Danubian Lowland, nearly parallel with Danube river.
The groundwater regime of the Žitný ostrov area is a result of interactions between Danube water (and other surface waters in the region) and groundwater in the region, as well as between precipitation and evaporation. Groundwater on the left bank of Danube River, border part of city is also the supplement of water from Malé Karpaty Mts. The Danube River during all its water levels in the Žitný ostrov feeds groundwater in the region. A general trend in the flow of groundwater is mostly following the main rivers in the region (Danube, Malý Dunaj and Váh). Precipitation is influencing groundwater regime especially during summer, in connection with elevated flow rates in rivers, and also by increasing the groundwater level (with different delay depending on the distance from the river). After operation of the Gabčíkovo water plant (monitoring between 1993 and 2002), groundwater levels have increased in the Bratislava region, at the upper part of the Žitný ostrov and in the direction to Malý Dunaj. The groundwater level is at a depth of 2–4 m.
Because the alluvial deposits are hydrogeochemically homogeneous, a uniform chemical type of groundwater develops throughout the area. This means that the chemical composition of groundwater changes from one place to another exclusively due to secondary agents, represented by anthropogeneous activities (Leerman et al. 1985; Ženišová et al. 2000). Compared to groundwater, the water in gravel pits is much more affected by secondary contamination because its water surface is open.
The water quality and its hygienic state are determinated by a number of hydrochemical, microbiological and biological parameters between which dominate faecal indicators of pollution represented by coliforms and enterococci. The presence of enterococci in waters is considered to be an important indicator of possible faecal contamination (Godfree et al. 1997). Our paper focuses to study water quality and possible chemical and microbiological contamination of gravel pits, some of which are used as natural swimming pools in surroundings of Bratislava, capital of Slovakia, by group of selected indicators.
Materials and methods
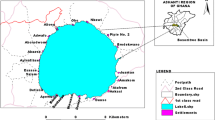
The water samples had been taken from eight gravel pits, and from single location of surface of Danube River. Three gravel pits on the right hand side of the Danube River (Čunovo, Rusovce and Draždiak), four gravel pits situated on the left hand side of the Danube River (Štrkovec, Kuchajda, Zlaté piesky and Vajnory) and one from Danube River (Fig. 1) were selected for monitoring.
The quality of water in gravel pits depends on the quality of water in the Danube River. Danube River samplings had been taken from right bank of the river in the up area of New Bridge around 1,869.5 km of the river.
The gravel pits, Rusovce and Čunovo, are situated nearest to the Danube River. Čunovo gravel pit is located in the protected area of water resource Rusovce-Ostrovné Lúčky-Mokraď at a distance of about 2 km from the Danube River. The gravel pit Rusovce is located about 700 m from the Danube River. The gravel pit Draždiak is located in the middle of the city suburban Petržalka, close to the housing developments, at a distance of 1.5 km from Danube River. The gravel pit, Štrkovec and Kuchajda, are located on the left bank of the Danube River at a distance of 3.5 km, respectively, 4.5 km from Danube River. Gravel pits, Zlaté Piesky and Vajnory, are located on the edge of the city, at a distance from Danube of River about 6 and 8 km, respectively (Fig. 1). There had been still some mining of the gravel in the gravel pit Vajnory in 2004 and 2005. The depth of water in gravel pits differs. It is mostly below 10 m. Locally there are deeper places. The deepest gravel pit is Zlaté piesky, with a depth more than 15 m.
The samplings had been taken 32 times to get chemical and microbiological analyses. Water sampling was realised according to EN ISO 5667-1 (2006) and ISO 5667-4 (1987). In the field, temperature of air, temperature of water, pH, dissolved oxygen, oxygen saturation, conductivity and redox potential, were measured. The pH was measured with a WTW Multi 350i using a electrode sentix 41; dissolved oxygen was measured with a WTW oximeter 340i/SET using a electrode DurOxR325-3 and the electrical conductivity was measured with a WTW Multi 350i using a electrode TetraConR325.
Water chemical analyses were made in the Hydrogeochemical Laboratory of the Department of Hydrogeology, Comenius University, and in the National Water Reference Laboratory for Slovakia. The following parameters were determined: CODMn, CODCr, BOD5, Na, K, Ca, Mg, Fe, Mn, NH4+, Cl−, NO3−, HCO3−, SO42−, HPO42−. All samples were kept at 4 °C until analyses. Sulphate was determined by gravimetry after precipitation with BaCl2 and Cl− by titration with Hg(NO3)2. Concentrations of NH4+, NO3−, and HPO43− were determined by spectrophotometry using a Perkin Elmer UV/VIS Lambda 11 apparatus. Na, K, Ca, Mg, Fe, Mn were analysed by AES-ICP (VISTA-MPX fy Varian).
Water samples for microbiological analysis were taken 2–3 m from bank in 100 mm depth. Samples were transported in aseptic 100 mL dark glass bottles in mobile ice box and treated within 24 h. Enterococci were isolated from 1 mL water which was spread directly on the surface of Slanetz–Bartley agar (Hi-Media), selective medium for enterococci and incubated for 44 h at 37 °C. Typical macromorphology of colonies (1–2 mm, red, maroon or pink) on Slanetz–Bartley agar were used for selection of enterococcal isolates for detailed molecular analysis at the genus and species level.
For molecular identification, the enterococcal chromosomal DNA was isolated by DNeasy purification kit (Qiagen). One bacterial colony resuspended in 50 μL water was used as template in Polymerase chain reaction (PCR). The PCR products were detected by electrophoresis in a 1.5 % agarose gel and stained with ethidium bromide. Tuf-PCR was used for identification of isolates belonging to genus Enterococcus according to protocol Ke et al. (1999). Fluorescent ITS-PCR: amplification of the internal spacer region (ITS) between 16S and 23S rRNA genes was used for species identification (Drahovská et al. 2002). Obtained ITS-PCR profiles of isolates were compared with the profiles of the standard reference strains for species classification. Panel of 18 reference strains was obtained from the Czech Collection of Microorganisms (CCM, Czech Republic), http://www.sci.muni.cz/CCM. The identification of E. faecalis and E. faecium isolates was confirmed by ddl-PCR (Dutka-Malen et al. 1995).
Results and discussion
The chemical composition of the Danube water, as the main source of infiltrated water to the alluvial deposits, has been constant for a long time (Ženišová et al. 2005) and the little changes in it are related to the seasonal minimum and maximum flows (Table 1, Ženišová et al. 2010). During the infiltration of water from the Danube River, its chemical composition changes not only with contacts of sediments but also with the effect of secondary factors such as escape form city’s canalization, waste deposits, pollution from chemical industry and so on.
Waters from gravel pits are contaminated by the dusty fall-out, rain fall, runoff from terrain and other pollution. Effect of these factors in each part of city is different and we can also see different levels of water contamination.
There are three groups of the gravel pits according to their chemical composition (Fig. 1). The first group is represented by the gravel pits Rusovce and Čunovo, this chemical composition is almost the same as the water in the river Danube, and are characterised by the lowest conductivity (Fig. 2). The second group is represented by the rest of the gravel pits with the exception of gravel pit Vajnory, where the quality of the water is significantly effected by surrounding city area. To compare with the water of the Danube River we would find significantly higher concentration of main ions, and much higher conductivity. The separate position belongs to the gravel pit Vajnory, that has had the highest conductivity, and the highest concentration of sulphate and chloride ions (Table 2).
The water in gravel pits has relatively high TDS (Total Dissolved Solids) and also conductivity (Fig. 2). TDS is ranging from 312 to 737 mg/L. Although, the TDS is partly influenced by the origin of water, but mostly by pollution sources. The lowest TDS was found in Čunovo gravel pit, which is situated nearest to the Danube River. There, the chemical composition is similar to the surface water and it is least secondary influenced. In contrast, the highest TDS was found in the Zlaté Piesky gravel pit, and in Vajnory gravel pit. These gravel pits are situated on the left side of the Danube, and are the farthest from the Danube River. Groundwater in this area forms through mixing of infiltrated Danube water with the water from Malé Karpaty Mts. There were documented the drop of sulphate, the highest in the gravel pit Čunovo, and chloride and nitrate, within the last 10 years. Generally, the quality of the water is getting better in all observed gravel pits. The Čunovo gravel pit is considered to be the least affected one, while the chemical composition of water in other gravel pits shows much stronger pollution.
Enterococci are chemoorgano-heterotrophs bacteria, widely distributed in nature, as commensals are occurring in intestinal tract of humans, mammals, birds, reptiles and insects (Devriese et al. 1992; Shosana and Manafi 1998). Enterococci as facultative pathogens are involved with high frequency in nosocomial infections and superinfections. Comparing with the clinical isolates, the species structure of aquatic enterococci is more complex and it is influenced by the type of contamination (Niemi et al. 1993; Svec and Sedlacek 1999).
Three PCR-based methods were used for identification of enterococcal isolates. Tuf-PCR with genus-specific primers was used for confirmation of isolates to belong into Enterococcus spp. Fluorescent ITS-PCR was further used for species identification. The internal spacer region between 16S and 23S rRNA genes was amplified and fluorescently labelled PCR products were separated by capillary electrophoresis, which enabled discrimination of DNA fragments up to 1 bp. To standardise the method, ITS-PCR profiles of reference strains belonging to 18 enterococcal species were analysed. The reference strains had species-unique profiles.
In all, 74 arbitrary selected presumptive isolates obtained from surface waters grown on Slanetz–Bartley agar were overall analysed. Thus, 46 isolates were positive in tuf-PCR, they were assigned to be enterococci. By ITS-PCR they were divided into five enterococcal species: E. faecium (20 isolates), E. faecalis (12 isolates), E. hirae (9 isolates), E. casseliflavus (4 isolates) and E.durans (1 isolate). 28 isolates were negative in tuf-PCR, the majority of these isolates were identified by ITS-PCR as Lactococcus garviae (genomospecies of E. seriolicida–9 isolates), L. lactis (8 isolates) and Aerococcus spp. (2 isolates). The rest of isolates remained unidentified because they did not amplify any product in ITS-PCR (7 isolates) or their ITS-PCR profiles did not match to any collection profile (2 isolates) (Table 3). We assume that in case of unidentified taxa, a close enterococcal species could be able to grow on selective Slanetz–Bartley agar, such as some streptococci.
The occurrence of enterococci in observed areas is significant to determinate of faecal contamination and quality of the water. Faecal enterococcal microflora (E.faecalis, E.faecium, E.durans, E.hirae) was isolated only from the river Danube (between 0 and 2.8 × 102 CFU/ml); and not from the gravel pit’s water. In those, we found out E.casseliflavus and E.seriolicida (between 0 and 1.3 × 101 CFU/mL). E.casseliflavus is isolated from different types of soils, and can penetrate to the water. E. seriolicida belongs to the category of terestric enterococci, and it is mostly only fish’s pathogen. That is why its occurrence in the gravel pits is not surprise; there is intensive fishery. Currently, E. seriolicida is genomically classified as a L. garviae, even it carries specific enterococcal phenotype characteristics (Teixeira et al. 1996). Besides the species mentioned above there were isolated types of lactococci and aerococci from the gravel pits, which are genetically close to enterococci and they create frequently their accompanying microflora.
We can explain the absence of the faecal enterococci in the gravel pits, even during the summer, by the relatively good self-cleaning ability of the environment. Low content of nutritious sources expressed as BOD5, CODMn, CODCr (Table 1) and predacious pressure do not create conditions suitable for reproduction of enterococci. The river Danube enters the territory of Bratislava with higher content of enterococci in the samples compared to the gravel pits, what could be explained by higher presence of organic matter in it. In conclusion, all mentioned above correlates with results of similar microbiological studies done by different authors (Ferley et al. 1989; Niemi and Niemi 1991; Niemi et al. 1993; Pinto et al. 1999; Svec and Sedlacek 1999, 2001).
References
Devriese LA, Collins MD, Wirth R (1992) The genus Enterococcus. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds) The prokaryotes: a handbook on the biology of bacteria: ecophysiology, isolation, identification, Applications, vol 2. Springer, New York, pp 163–195
Drahovská H, Kocíncová D, Seman M, Turňa J (2002) PCR-based methods for identification of Enterococcus species. Folia Microbiol 47:649–653
Dutka-Malen S, Evers S, Courvalin P (1995) Detection of glycopeptides resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol 33:24–27
EN ISO 5667-1 (2006) Water quality. Sampling. Part 1: Guidance on the design of sampling programmes and sampling techniques
Ferley JP, Zmirou D, Balducci F, Baleux B, Fera P, Larbaigt G, Jacq E, Moissonier B, Blineau A, Boudot J (1989) Epidemiological significance of microbiological pollution criteria for river recreational waters. Int J Epidemiol 18:198–205
Godfree AF, Kay D, Wyer MD (1997) Fecal streptococci as indicators of fecal contamination in water. J Appl Microbiol 83:110S–119S
ISO 5667-4 (1987) Water quality. Sampling. Part 4: Guidance of sampling from lakes, natural and man-made
Ke D, Picard FJ, Martineau F, Ménard Ch, Roy PH, Ouellette M, Bergeron MG (1999) Development of a PCR assay for rapid detection of enterococci. J Clin Microbiol 37:3497–3503
Leerman A, Imboden D, Gat J (1985) Physics and chemistry of lakes. Springer, Berlin
Mucha I, Lisický MJ (eds) (2006) Slovak-Hungarian environmental monitoring of the Danube. Ground Water Consulting Ltd., Bratislava
Mucha I, Kocinger D, Hlavatý Z, Rodák D, Banský Ľ, Lakatosová E, Hučárová K (2004) Gabčíkovo Water Plant and the environment. Ground Water Consulting Ltd., Bratislava (in Slovak)
Niemi RM, Niemi S (1991) Bacterial pollution of waters in pristine and agricultural lands. J Environ Qual 20:620–627
Niemi RM, Niemilä SI, Bradford DH, Hantula J, Hyvärinen Y, Forsten T, Raateland A (1993) Presumptive faecal streptococci in environmental samples characterized by one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Appl Environ Microbiol 59:2190–2196
Pinto B, Pierotti R, Canale G, Reali D (1999) Characterization of “faecal streptococci” as indicators of faecal pollution and distribution in the environment. Lett Appl Microbiol 29:258–263
Shosana BD, Manafi M (1998) Use of enzymes test in characterization and identification of aerobic and facultatively anaerobic gram-positive cocci. Clin Microbiol Rev 11:318–340
Svec P, Sedlacek I (1999) Occurence of Enterococcus spp. in waters. Folia Microbiol 44:3–10
Svec P, Sedlacek I (2001) Molecular taxonomy of enterococci isolated from water. Bull Ceskoslov spol mikrobiol 17:66–67 (in Czech)
Teixeira LM, Merquior VLC, Vianni MCE, Carvalho MGS, Fracalanzza SEL, Steigerwalt AG, Brenner DJ, Facklam RR (1996) Phenotypic and genotypic characterization of atypical Lactococcus garvieae strains isolated from water buffaloes with subclinical mastitis and confirmation of Lactococcus garvieae as a senior subjective synonym of Enterococcus seriolicida. Int J Syst Bacteriol 46:664–668
Ženišová Z, Fľaková R, Roháčiková A (2000) The organic compounds in waters of gravel pits of Bratislava. Podzemna voda 6:185–192 (in Slovak)
Ženišová Z, Panák D, Fľaková R, Seman M (2005) Hydrogeochemical and microbiological characteristic of gravel pits in surroundings of Bratislava. Podzemna voda 11:178–188 (in Slovak)
Ženišová Z, Dobrovoda D, Šutarová B, Ľuptáková A, Bodácz B (2010) Groundwater chemical composition changes in monitoring well kalinkovo in the Žitný ostrov area. Podzemna voda 19:127–147 (in Slovak)
Acknowledgement
This study was financially supported by the Slovak Grant Agency, project VEGA No. 1/0117/09.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Fľaková, R., Seman, M., Drahovská, H. et al. The Water quality of the Danube River and gravel pits in the Bratislava area (Slovakia). Int Aquat Res 6, 203–210 (2014). https://doi.org/10.1007/s40071-014-0079-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40071-014-0079-1