Abstract
The Asian striped catfish Mystus vittatus (Bloch) are exposed to ZnS nanoparticles of different concentrations and its impact on feeding behaviour, growth and maturity of the fish is studied. The study reveals the fact that under nanoparticle exposure, the feeding behaviour, growth and maturation stages depart from that of the controlled conditions. The growth is found to be restricted with increasing nanoparticle concentration. Gonadal maturity has also found to be constrained with increasing nanoparticle concentrations up to a certain level. These effects are found to be more pronounced for nanoparticles of smaller sizes. The observations are explained on the basis of the enhanced photo-oxidation property of the ZnS nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The expansion of nanoparticle (1–100 nm) research has resulted in an increasing number of consumer and therapeutic products containing nanomaterials (Masciangioli and Zhang 2003; Shipway et al. 2000). Due to the wide applications of nanoparticles, human and environmental exposures of these materials are gradually becoming more likely. Because of their very small size, nanoparticles have chemical properties that differ from those of their bulk counterparts. Till now, not much knowledge is acquired regarding human or environmental health outcomes following exposure to nanoparticles. Recently, nanoparticles have come under scrutiny for their potential to cause environmental damage (Nowack and Bucheli 2007; Krysazenov et al. 2010). Therefore, it is very important to understand the potential impacts of nanoparticles upon biotic communities and their environments.
The increased production and wide applications of nanoparticles are making it more likely that such materials will end up in watercourses, either as medical or industrial waste, or when used as ecological tools with unknown consequences for aquatic life. Recent studies (Smith et al. 2007; Oberdorster 2004; Koziara et al. 2003; Zhu et al. 2006; Bhattacharjee et al. 2013; Griffitt et al. 2007; Fedirici et al. 2007) (5–11) have thrown some light on the adverse effect of nanoparticles on aquatic fauna. Carbon nano-tubes are found to be a respiratory toxicant in rainbow trout (Smith et al. 2007). Fullerenes (C60) are shown to be detrimental for aquatic environments causing oxidative damage in largemouth bass (Micropterus salmoides) (Oberdorster 2004) by acting through the same mechanism of action found to be beneficial for their use in drug delivery (Koziara et al. 2003). Fullerenes are found to travel to the brain, bind with lipids, and cause the production of oxidative stress compounds when fish are exposed to concentrations of only 1 ppm (Oberdorster 2004). Fullerenes are found to interact adversely with zooplankton and fish (Zhu et al. 2006). ZnS nanoparticles are shown to be harmful for Daphnia sp. (Bhattacharjee et al. 2013) by reducing the dissolved oxygen content in water due to enhanced surface photo-oxidation associated with its nanoparticle nature. It is found that copper nanoparticles harm gills of Zebra fish (Danio rerio) through an unknown mechanism, which is different from that of dissolved copper ions (Griffitt et al. 2007). Exposure of TiO2 nanoparticles to rainbow trout (Oncorhynchus mykiss) (Fedirici et al. 2007) is found to result in respiratory problems and other sublethal effects in the fish.
In different districts of West Bengal (a state in India), some of the industries are cropping up without having proper site for sewage disposal. The wastes of these industries contain predominantly sulphide nanoparticles. ZnS is one of such materials that can be found in the wastes of cosmetic, pharmaceutical and rubber industries. ZnS possess the property of photo-oxidation. Apart from the direct effect of various physiological disorders due to ingestion of nanoparticles by the aquatic animals, ZnS nanoparticles are expected to exhibit some passive effects on aquatic ecosystem by reducing the dissolved oxygen content of water due to its property of surface photo-oxidation. This effect can be more pronounced for nanoparticles of smaller sizes having greater surface area.
Fish is one of the major sources of edible protein in India. Therefore, its reproduction has acquired prime importance to the investigators working in this area. Mystus vittatus is a species of freshwater catfish, found mainly in the rivers of India, Bangladesh and Myanmar. Recently, it has been reported as a vulnerable species in the Indian waters. This species is easy to cultivate and it is an important target species for the small-scale fishermen, who use a variety of traditional fishing gear (Craig et al. 2004; Kibria and Ahmed 2005). This small indigenous fish species has a high nutritional value in terms of protein, micronutrients, vitamins and minerals not commonly available in other foods (Ross et al. 2003) making it a very attractive candidate for aquaculture in the South East Asia. Being a vulnerable species with immense economical importance, M. vittatus demands special attention to be monitored against the impact of ZnS nanoparticles on it.
The aim of our present study is to monitor systematically the adverse effect of ZnS nanoparticles on M. vittatus regarding their feeding behaviour, growth and maturity. The changing behaviour in growth and maturity of any member of an aquatic environment due to exposure of nanoparticles may cause an adverse effect on the aquatic ecosystem. This in turn may affect the human race as a whole. Therefore, it is gradually becoming very important to identify the most appropriate route of nanotechnology that will preserve the aquatic environment while also advancing industrial, medical and environmental technology. The present study will also help in that purpose.
Experimental
A simple wet chemical technique (Chen et al. 1997) is employed to synthesize ZnS nanoparticles. A solution of Zn(NO3)2·6H2O (purified, Merck India) dissolved in 2-propanol, (CH3)2CHOH (GR, Merck India) dried over activated molecular sieve zeolite 4A and distilled water is used as the zinc precursor. The volume ratio of water and alcohol is 2-propanol:H2O = 1:5 which is maintained throughout to prepare the solution. This solution is stirred for 2 h. In another solution, sodium sulphide (Na2S) (purified, Merck India) is dissolved in distilled water and also stirred for 2 h. This solution is used as the sulphur precursor. The precursors are so chosen that the molar ratio of zinc and sulphur is maintained as Zn:S = 1:1 to obtain the required stoichiometry in the derived zinc sulphide. After stirring, Na2S solution is quickly injected to the solution containing Zn precursor. Immediate formation of colloids containing ZnS nanoparticles is observed. The particle size is controlled by varying the reaction temperature. Once the reaction is completed, the solution is cooled to room temperature. The precipitates are centrifuged, washed with de-ionized water, ethanol and acetone several times and dried at 50 °C in a vacuum oven and kept in vacuum for further use.
Transmission electron microscopy (TEM) is performed using a Hitachi H-7100 microscope operated at the voltage of 100 kV. Powder dispersed in ethanol is carefully placed on the carbon coated Cu grid for TEM study. A particle size analyzer (Nano-S, Malvern, Worcestershire, UK) is used to obtain the size distribution histogram using methanol as the solvent. X-ray diffraction (XRD) study is performed in a MAC M03 XHF diffractometer using Ni filtered Cu K α radiation (λ = 0.154056 nm) as X-ray source at 40 kV, 30 mA. The θ/2θ scans are carried out at a scanning speed of 2°/min in the 2θ range of 20°–60°. Energy dispersive X-ray (EDX) study is performed in the instrument Hitachi S-2400. X-ray photoelectron spectroscopic (XPS) measurements are performed on commercial VG Microtech (MT-500) machine using Al K α radiation. The C1s peak located at 284.6 eV is taken as the internal standard for all samples and all the peak positions are normalized with respect to it. Charge correction is calibrated from the observed C1s binding energy.
Asian striped catfish specimens of different size, maturity and sex groups are collected randomly in monthly basis from different places of Jalpaiguri, Hooghly, Burdwan, Bankura and Purulia districts of West Bengal, India, during the period of September, 2008–August, 2012 during daytime (1,000–1,700 h) by means of traditional fishing gear cast net and conical trap. After collection, fishes are kept in watertight containers containing tap water that has been allowed to stand for a few days. A good supply of necessary oxygen is provided by using a large shallow tank to ensure that a large surface area of water is exposed to the air. Fishes are maintained at 25–30 °C of temperature to ensure a natural environment. The fishes are fed on natural fish foods. Small, regular supplies of food are provided. The fishes are filtered out every day and are placed in waters containing freshly prepared ZnS nanoparticles.
To study the feeding behaviour, fishes are washed, cleaned and then the quantitative analysis of stomach contents of each fish is done. For studying the growth behaviour, lengths of the fishes are measured with a slide caliper of the vernier constant of 0.01 cm, while the body weights and gonad weights are determined with a digital balance to the nearest 0.001 g.
To study the liver histology, liver tissues are dissected out and cut into small pieces for preservation in Bouin’s fixative for 18 h. The tissues are then dehydrated through ethanol, C2H5OH (GR, Merck India) dried over activated molecular sieve zeolite 4A, cleared in xylene and embedded in paraffin of melting point 56–58 °C. Thin sections of 4 µm thicknesses are cut using a rotary microtome machine. The sections are stained with Delafield’s haematoxylin and eosin stain and are observed under a light microscope.
A properly calibrated electronic lab meter with a probe is used to measure the dissolved oxygen content in water. A calibration curve is drawn and used to obtain the dissolved oxygen content in water under different experimental conditions.
Fish specimens are exposed to seven concentrations (50, 100, 200, 250, 500, 750 and 1,000 μg/L) of the ZnS nanoparticles of different sizes (3, 7, 12 and 20 nm) whole day throughout the year. Trials are conducted at various concentrations to observe effects and to obtain the concentration that caused maximum deviation from the controlled condition. After this range is achieved, trials are repeated to observe the effect of time of exposure of ZnS nanoparticles on M. vittatus. Impact of ZnS nanoparticles on M. vittatus are characterized through comparing the feeding behaviour, liver histology, length–weight relationship, condition factor (K) and gonado-somatic index (GSI) of the exposed fishes to that of the fishes lived in controlled conditions.
Results
Microstructures of ZnS nanoparticles
Figure 1a shows the TEM of ZnS nanoparticles with the corresponding diffraction pattern in inset. Presence of fine ZnS nanoparticles is clearly visible in the TEM picture (Fig. 1a). The diffraction pattern of the sample consists of a central halo with concentric broad rings. The rings correspond to the reflections from (111), (220) and (311) planes confirming the cubic crystallographic structure of the ZnS nanoparticles. The average size (dav) of the nano-crystallites determined from TEM is around 12 nm (±0.5 nm). Particle size analysis (PSA) data shows (Fig. 1b) narrow size distribution of the particles with dav 12 nm. This result is in confirmatory with the TEM result. Figure 1c illustrates a representative XRD pattern of ZnS nanoparticles. The pattern showed peak from (111), (220) and (311) planes, indicating the formation of cubic phase, in agreement with the electron diffraction results. Broadening of the XRD peaks can be attributed to the small size of the ZnS nanoparticles present in the sample. The crystallite size was obtained as 12.16 nm from the XRD data using the Debye equation. This value tallied well with the TEM and PSA results as mentioned earlier.
Compositional analysis (EDX and XPS) of the ZnS nanoparticles
The chemical composition of ZnS nanoparticles are determined by EDX measurements. The Zn/S ratio for the samples with different particle sizes is obtained from EDX data. To determine the chemical uniformity of the nanoparticles, EDX are done at different parts of the samples and an average for each sample is taken (Table 1). The data clearly reveals almost uniform chemical homogeneity (Zn/S ratio ~1) of the ZnS nanoparticles.
The EDX data are further verified performing XPS. Figure 2 shows the typical XPS survey spectrum of representative ZnS nanoparticles with particle size of 12 nm with corresponding Zn 2p and S 2p core-level spectra. All the photoelectron and Auger peaks in the survey spectrum are identified and attributed to different levels of Zn and S. The positions of these peaks are found to be in agreement with the reported literature (Nanda and Sarma 2001). Existence of very trace amount of C and O in the survey spectrum can be attributed to the adventitious carbon and oxygen contamination due to atmospheric exposure of the sample. The peak areas of the Zn 2p and S 2p core levels are measured and used to calculate the atom percent of Zn and S for the different samples. Elemental composition is calculated using the following relationship:
where A x is the area (or intensity of the peak) under the curve for the element x and S x is the corresponding sensitivity factor. Quantitative analysis results, as shown in Table 2, indicated that the Zn:S ratios in the samples are very close to 1:1. Thus, the Zn/S ratio calculated from XPS data tallies well with that of EDX data confirming stoichiometry of the samples.
Impact of ZnS nanoparticle exposure on feeding behaviour
The gut content analysis of fishes for both the sexes throughout the year shows that the fish feed on a variety of food items. The feeding behaviour of the fish is quantized through calculating the parameter, percentage of non-empty stomach, defined as follows
The results of the study of the seasonal feeding patterns of female fishes without nanoparticle exposure have been presented in Fig. 3a. It is observed that during the pre-spawning period of April–May, the fish minimize its feeding rate. Later in August and September, a marked rise in the feeding rate is observed. Same type of feeding behaviour is observed for male M. vittatus. Begum et al. (2008) reported a similar type of observation from their studies on the feeding intensity of M. gulio in the south-west coast of Bangladesh. Reddy and Rao (1987) studied the food of M. vittatus and observed seasonal variation in the rate of feeding. They recorded no uniform pattern in the 2 years of study. The maximum rate of active feeding was observed by Bhatt (1971) during December–February in M. vittatus from Aligarh.
Seasonal variation of the percentage of non-empty stomach of female Mystus vittatus under exposure of increasing concentration of ZnS nanoparticles with particle size of 3 nm: a concentration of 0 μg/L, b concentration of 100 μg/L, c concentration of 250 μg/L and d concentration of 500 μg/L, inset variation in the percentage of non-empty stomach with increasing concentration of 3 nm ZnS nanoparticles due to exposure time of 72 h in the month of September
Figure 3b–d shows the effect of increasing nanoparticle (d = 3 nm) concentration on the average feeding behaviour of female M. vittatus throughout the year. Departure in feeding behaviour from controlled condition can be seen clearly from these figures. Figure 3d (inset) shows that the variation of percentage of non-empty stomach with increasing nanoparticle concentration for fixed nanoparticle size (3 nm) and exposure time (72 h) in a particular month (September). The month of September is chosen for this analysis due to the reason that the maximum percentage of non-empty stomach in the fishes is observed in controlled condition in this month. The values of percentage of non-empty stomach is found to decrease with increase in nanoparticle concentration up to 500 μg/L. Beyond this concentration, this value remains nearly constant. This observation gradually fades out when nanoparticle size is increased from 3 to 7, 12 and 20 nm. Similar qualitative variation is found for male M. vittatus.
Impact of ZnS nanoparticle exposure on liver histology
The liver cell structure of teleosts responds very sensitively to environmental changes, e.g. in temperature, season, feeding conditions or presence of various chemicals in the water (Segner and Möller 1984). Therefore, liver histology can be used as an indicator to show the harmful effect of ZnS nanoparticles on M. vittatus. In controlled condition, the liver cells are expected to be found in normal and healthy states unlike those who are exposed to ZnS nanoparticles of different concentrations.
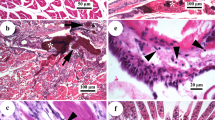
Figure 4a shows the liver cells of a representative female fish having no nanoparticle exposure. In this figure, liver cells are found to be large with regular outlines. These cells are dominated by storage deposits. The nuclei are found to be large and centrally located indicating the normal condition of the cells. The cells are found to be in close contact, almost no empty space is found between the cells.
Liver histology of female Mystus vittatus under exposure of increasing concentration of ZnS nanoparticles with particle size of 3 nm: a concentration of 0 μg/L, b concentration of 100 μg/L, c concentration of 250 μg/L and d concentration of 500 μg/L, in the month of September (N nucleus, CV central vein, ES empty space, H hepatocytes)
Figure 4b–d shows the effect of increasing nanoparticle (d = 3 nm) concentration on the liver histology of female M. vittatus in the month of September. The month of September is chosen because maximum percentage of non-empty stomach in the fish is found in this month for controlled condition as discussed in “Impact of ZnS nanoparticle exposure on feeding behaviour”. For exposure to ZnS concentration of 100 µg/L (Fig. 4b), few cells are found to be in degenerating states without a prominent nucleus and having diffused cytoplasmic contents. For higher concentration of ZnS nanoparticles, decrease in cell sizes due to drastic loss of storage deposits is observed (Fig. 4c, d). Therefore, the relative share of nucleus in cell volume is strongly increased. The cells are found to be in increasing isolated states having no close contact between them. These effects are more pronounced for higher nanoparticle concentration. The observation is similar for male M. vittatus. These observations are indicative of degradation of liver cells under nanoparticle exposure and can be associated directly with the changing feeding behaviour, which in turn makes a detrimental effect on growth and maturity of the fish.
Impact of ZnS nanoparticle exposure on the growth
Normal growth of M. vittatus (Bloch) throughout the year can be divided into three periods: (a) pre-spawning, (b) spawning and (c) post-spawning. The growth process of the fish can be monitored systematically using length–weight relationships (LWRs). LWRs are useful in fishery management for both applied and basic use (Pitcher and Hart 1982). It can be employed to: (1) estimate weight from length observations; (2) calculate production and biomass of a fish population. This also provides information on stocks or organism condition at the corporal level. The LWR can be obtained from length and weight measurements of the same fishes throughout their lives or from a sample of fish taken at a particular time (Wootton 1990). In the present study, the lengths of the fishes are taken to be ranging between 40 and 180 mm. No specimens with length <30 mm are used in LWR estimations to avoid potential bias of the inclusion of immature juveniles that had not yet attained adult shape (Bagenal and Tesch 1978). The weight (W) in gram and length (L) in cm are assumed to obey the relation
In this equation, the coefficient “a” is the intercept in the y axis and the regression coefficient “b” is an exponent indicating isometric growth when equal to 3. The statistical significance level of r2 and the parameters a and b are estimated by linear regressions on the transformed equation
Figure 5a shows plots for the female fishes lived in controlled conditions whereas Fig. 5b–d is for those exposed to ZnS nanoparticles of 3 nm size for different concentrations throughout the year. The data plotted are taken in the month of June. The month of June is chosen for this analysis because this month shows the peak in the spawning process of the fish. Analysis of covariance revealed significant differences between the slopes (b) of the regression lines (p < 0.001) for nanoparticle exposures of different concentrations. All allometric coefficients (b) estimated in this study are found within the range of 2.7–3.1, though they can vary between 2 and 4 (Bagenal and Tesch 1978). The b value of the overall LWR for female fishes not exposed to ZnS nanoparticles is found to be close to the isometric value (b = 3.051), although it suggests negative allometric growth for female fishes exposed to ZnS nanoparticles with decreasing b value for increasing nanoparticle concentration up to 500 μg/L for which b = 2.769 (Fig. 5d, inset). Beyond this concentration, b value remains fairly constant. The experiment also suggested negative allometric growth in male fishes with decreasing b value for increasing nanoparticle concentration from b = 3.089 for unexposed fishes to b = 2.807 for exposure to ZnS nanoparticles of concentration of 500 μg/L.
Relationship between length (L) and body weight (W) for female Mystus vittatus in the month of July under exposure of increasing concentration of ZnS nanoparticles with particle size 3 nm: a concentration of 0 μg/L, b concentration of 100 μg/L, c concentration of 250 μg/L and d concentration of 500 μg/L, inset variation in b value as a function of increasing nanoparticle concentration for the month of June
Condition factor is a quantitative parameter of the state of well-being of a fish. This determines present and future population success by its influence on growth, reproduction and survival. The Fulton’s condition factor (K) is calculated for each individual fish according to the equation
The Fulton’s condition factors (K) are found to be ranged 0.968–3.281 in fishes that are not exposed to the ZnS nanoparticles. No significant differences in K value are found between months (p > 0.05). The average K value in females (K = 2.219) is found to be significantly higher than that in males (K = 2.026). In females, the lowest average K is found in April (K = 1.581) and the highest in August (K = 3.281). In males, the lowest average K is found in April (K = 0.968) and the highest found in August (K = 3.178). After the fishes are exposed to nanoparticles of size 3 nm at a concentration of 100 μg/L for 1 year, the average K value is found to be K = 2.011 in females and K = 1.896 in males. Both the values are significantly lower than that of the controlled condition. The average K value in both females and males show further reduction with increase in nanoparticle concentration up to 500 μg/L. Beyond this concentration, the average K value remains nearly constant for both males and females. This effect gradually loses its prominence with increasing nanoparticle size.
Impact of ZnS nanoparticle exposure on the gonadal maturity
The parameter GSI is employed to determine gonadal maturity. This is defined by the equation given by
In normal condition, the GSI for both males and females rapidly increase between March and April, maintaining a high level until July and then rapidly decrease. In April M. vittatus may start the reproductive period, on the other hand in August the fish may already recover. The spawning season can be estimated to continue from March to August, with a peak during June. When the fishes are exposed to ZnS nanoparticles throughout the year, no significant change is observed in the seasonal variation of the GSI for male fishes (p > 0.05), but the impact of ZnS nanoparticles on GSI of female fishes can be clearly observed (p < 0.001).
Figure 6 shows the change in GSI with increasing nanoparticle (d = 3 nm) concentration with for female fishes throughout the year. It can be seen from the figure that the seasonal variation of GSI remains qualitatively same, but its maximum value at June decreases from 18.54 for unexposed fishes to 14.6 for exposure to nanoparticle concentration of 100 μg/L, 9.17 for concentration of 250 μg/L and 6.22 for concentration of 500 μg/L (Fig. 6, inset). Beyond this concentration, the GSI value remains nearly constant. This effect gradually loses its prominence with increasing nanoparticle size.
Seasonal variation in the gonado-somatic index (GSI) value in female Mystus vittatus under exposure of increasing concentration of ZnS nanoparticles with particle size of 3 nm: a concentration of 0 μg/L, b concentration of 100 μg/L, c concentration of 250 μg/L and d concentration of 500 μg/L, inset variation in GSI value with increasing concentration of ZnS nanoparticles of particle size of 3 nm in the month of July
Discussions
Due to the enhanced surface photo-oxidation property associated with the greater surface area, ZnS in the nanoparticle form poses serious threat to the aquatic lives by reducing the dissolved oxygen content in the water. Dissolved oxygen content in some water body acts as an important factor related to the metabolism and growth for fishes living in that water (Kutty 1968; Hart 1947; Hoar 1966). With reducing oxygen level, swimming activity and therefore migration and even feeding activity of fishes are observed to be restricted (Kutty 1968; Hart 1947). When oxygen level is reduced to a point which can sustain only the basal requirement of the fish, it cannot afford any further physical activity at that specific oxygen level. Below this level (lethal or asphyxial level of oxygen), oxygen becomes inadequate and the fishes die due to asphyxiation (Hoar 1966).
In the present study, the dissolved oxygen content in water is measured to be 13.2 mg/L at 15 °C before any nanoparticle is introduced in it. This value is found to decrease with increasing exposure time of the nanoparticles in water at the same temperature. Figure 7a shows the variation of the dissolved oxygen content in water as a function of nanoparticle exposure time for nanoparticles of different sizes with the concentration of 500 µg/L. It is also clear from this figure that the rate of reduction in dissolved oxygen content is higher for the nanoparticles with smaller sizes.
a Variation of dissolved oxygen content in water at 15 °C with nanoparticle exposure time for ZnS nanoparticles with different sizes having concentration of 500 μg/L, b X-ray photoelectron spectra of S 2p core level of ZnS nanoparticles with particle size of 3 nm after different period of exposure in water under sunlight
The decrease in the dissolved oxygen content in water due to exposure of ZnS nanoparticles can be explained by the instability of ZnS due to photo-oxidation. It is an indication of the surface oxidation of the nanoparticles when it is exposed to visible light in a humid atmosphere. Lu et al. (2006) have studied the photo-oxidation properties of uncapped CdSe nanoparticles in humid atmosphere using XPS data. A similar study on XPS spectra of S 2p core level of ZnS nanoparticles (d = 3 nm) after water exposure (Fig. 7b) has revealed the fact that due to photo-oxidation, the surfaces of the ZnS nanoparticles get oxidized using the dissolved oxygen in water in the presence of sun light. This can be confirmed by the formation of peak related to SO2 at energy value nearly 168 eV after 2 days of exposure. This peak is at an energy value higher than that of the main S 2p peak (~162.8 eV) related to ZnS.
It is interesting to note the temporal behaviour of the peak related to SO2 formation with increasing exposure time. The initial rise of this peak indicates the increasing concentration of chemisorped SO2 at ZnS surface with increasing exposure time. After that, the peak decays indicating that the oxide leaves the surface as a molecular species (SO2), leaving Zn and a freshly exposed layer of ZnS behind. Water may dissolve a part of the SO2 released in the process causing reduction in the pH value of the water to 6.3. Then the peak is found to rise again. Oscillations continued over the period of several days. During the period of decaying of the oxidized peak, the Zn/S ratio is found to rise over that of the original value of the freshly prepared samples confirming the loss of S from the nanoparticles. Surface of the nanoparticles of ZnS exposed to water and light is thus effectively destroyed by these redox cycles within a few days and results in the reduction of the dissolved oxygen content in water.
The positions of S 2p peaks related to ZnS for all the samples are found to be blue shifted (~162.8 eV) when compared to that of the freshly prepared ZnS samples (~162 eV), after water exposure under sunlight. This can be seen in Fig. 7b for ZnS nanoparticles of 3 nm size. This observation is possibly due to the photoinduced adsorption of oxygen on ZnS surface, which is also responsible for reducing the dissolved oxygen content in water. Photoinduced adsorption of oxygen on ZnS surface has been reported (Becker and Bard 1983) for colloidal ZnS dispersions after photo irradiation in the presence of oxygen in the solution. Kobayashi and Kawaji (1955) attribute the removal of atmospheric oxygen to photo-enhanced adsorption on ZnS surface for dry ZnS powders. Chemisorption of oxygen on ZnS has been said to proceed by both thermal and radiative processes; in thermal adsorption O2 is said to be in the form of the ion O2−, while in the radiative chemisorption, the form O− is proposed (Muminov et al. 1975). Oxygen consumption from irradiated ZnS colloidal suspensions probably occurs by the similar photo-enhanced adsorption process (Becker and Bard 1983). The presence of oxygen or an oxide species on the surface of the colloidal semiconductors should have a profound effect on surface recombination of photogenerated electrons and holes resulting in the observed blue shift of XPS peaks.
The increasing deficiency in dissolved oxygen content with increasing nanoparticle concentration or exposure time can be attributed to the hazardous effects of changing feeding behaviour (“Impact of ZnS nanoparticle exposure on feeding behaviour”), degraded hepatic morphohistology (“Impact of ZnS nanoparticle exposure on liver histology”), restricted growth (“Impact of ZnS nanoparticle exposure on the growth”) and constrained gonadal maturity (“Impact of ZnS nanoparticle exposure on the gonadal maturity”) in M. vittatus under exposure of ZnS nanoparticles. The lethal level of oxygen content for M. vittatus is not achieved during this experiment. This results in recording of zero mortality in the fish due to oxygen deficiency though the decreasing oxygen content is found to affect hazardously on the fish feeding behaviour, growth and maturity. The lethal level of oxygen is found to vary markedly for different fish species (Davis 1975). Fishes which live in cold waters such as salmonids (salmon and rainbow trout) have a high lethal level of oxygen, but tropical fishes such as tilapias and carps tolerate very low value of lethal oxygen levels (Davis 1975; Kutty 1972). Being a catfish of tropical region, M. vittatus is supposed to tolerate a very low lethal level of oxygen that is not achieved in the present study.
The detrimental effect in growth and maturity is found higher for exposure of the fishes to the smaller particles. This observation can be explained by the fact that smaller particle size culminates in higher surface-to-volume ratio of the nanoparticles present in the water. ZnS nanoparticles having smaller sizes offer greater surface area. This made the particles more sensitive to surface photo-oxidation process, leading to a faster deficit in dissolved oxygen content compared to the samples having larger particle sizes.
Conclusion
As the Asian striped catfish M. vittatus (Bloch) are exposed to the ZnS nanoparticles of different concentrations, the feeding behaviour of the fish is found to be departed from that under the controlled conditions. The growth is found to be restricted with increasing nanoparticle concentration. Gonadal maturity is also found to be hampered with increasing nanoparticle concentration up to a certain level. These effects are found to be more pronounced for nanoparticles with smaller sizes. These observations are explained on the basis of the enhanced photo-oxidation property of the ZnS nanoparticles.
References
Bagenal TB, Tesch FW (1978) Age and growth. In: Begenal T (ed) Methods for assessment of fish production in fresh waters, 3rd edn. IBP Handbook No. 3, Blackwell Science Publications, Oxford, pp 101–136
Becker WG, Bard AJ (1983) Photoluminescence and photoinduced oxygen adsorption of colloidal zinc sulphide dispersions. J Phys Chem 87:4888–4893
Begum M, Alam MJ, Islam MA, Pal HK (2008) On the food and feeding habit of an estuarine catfish (Mystus gulio, Hamilton) in the south-west coast of Bangladesh. Univ J Zool Rajshahi Univ 27:91–94
Bhatt VS (1971) Studies on the biology of some freshwater fishes Part V. Mystus vittatus (Bloch). J Bombay Nat Hist Soc 68(3):556–572
Bhattacharjee B, Chatterjee N, Lu CH (2013) Harmful impact of ZnS nanoparticles on Daphnia sp. in the western part (districts of Bankura and Purulia) of West Bengal, India. ISRN Nanomater. doi:10.1155/2013/207239
Chen W, Wang Z, Lin Z, Lin L (1997) Absorption and luminescence of the surface states in ZnS nanoparticles. J Appl Phys 82(6):3111–3115
Craig JM, Halls AS, Barr JJF, Bean CW (2004) The Bangladesh floodplain fisheries. Fish Res 66:271–286
Davis JC (1975) Minimal dissolved oxygen requirements of aquatic life with emphasis on Canadian species: a review. J Fish Res Board Can 32:2295–2332
Fedirici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress and other physiological effects. Aquat Toxicol 84:415–430
Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D, Barber DS (2007) Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ Sci Technol 41:8178–8186
Hart JS (1947) Lethal temperature relations of certain fish of the Toronto region. Trans R Soc Can 41:57–71
Hoar WS (1966) General and comparative physiology. Prentice-Hall, Englewood Cliffs
Kibria G, Ahmed KKU (2005) Diversity of selective and nonselective fishing gear and their impact on inland fisheries in Bangladesh. NAGA 28:43–48
Kobayashi A, Kawaji S (1955) Adsorption and surface potential of semi-conductors. Part 1. Photo-enhanced adsorption of oxygen and change of contact potential of ZnS phosphors with illumination. J Phys Soc Jpn 10:270–273
Koziara JM, Lockman PR, Allen DD, Mumper R (2003) In situ blood–brain barrier transport of nanoparticles. J Pharmacol Res 20:1772–1778
Krysazenov E, Pavlov D, Demidova T, Dgebuadze Y (2010) Effect of nanoparticles on Aquatic organisms. Biol Bull Russl Acad Sci 37:406–412
Kutty MN (1968) Influence of ambient oxygen on the swimming performance of goldfish and rainbow trout. Can J Zool 46:647–653
Kutty MN (1972) Respiratory quotient and ammonia excretion in Tilapia mossambica. Mar Biol 16:126–133
Lu CH, Bhattacharjee B, Hsu CH, Chen SY, Ruaan RC, Chang WH (2006) Highly luminescent CdSe nanoparticles embedded in silica thin films. J Electroceramics 17:21–29
Masciangioli T, Zhang W-X (2003) Environmental technologies at the nanoscale. Environ Sci Technol 37:102A–108A
Muminov MI, Kim GCh, Zaitov FA, Kalamozov RU (1975) Some features of the chemisorption of oxygen on semiconductors with different widths of the forbidden band. Dokl Akad Nauk Uzb SSR 12:20–21
Nanda J, Sarma DD (2001) Photoemission spectroscopy of size selected zinc sulphide nanocrystallites. J Appl Phys 90(5):2504–2510
Nowack B, Bucheli TD (2007) Occurence, behaviour and effects of nanoparticles in the environment. Environ Poll 150:5–22
Oberdorster E (2004) Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in brain of juvenile largemouth bass. Environ Health Perspect 112:1058–1062
Pitcher TJ, Hart PJ (1982) Fisheries ecology. Chapman and Hall, London
Reddy YS, Rao MB (1987) A note on the food of Mystus vittatus (Bloch) from the highly polluted Hussain Sagar lake Hyderabad. Indian J Fish 34(4):484–487
Ross N, Islam M, Thilsted SH (2003) Small indigenous fish species in Bangladesh: contribution to vitamin A, calcium and iron intakes. J Nutr 133:4021–4026
Segner H, Möller H (1984) Electron microscopical investigations on starvation induced liver pathology in flounders Platichthys flesus. Mar Ecol Prog Ser 19:193–196
Shipway AN, Katz E, Willner I (2000) Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. Chem Phys Chem 1:18–52
Smith CJ, Shaw BJ, Handy RD (2007) Toxicity of single walled carbon nanotubes to rainbow trout, (Oncorhynchus mykiss): respiratory toxicity, organ pathologies, and other physiological effects. Aquat Toxicol 82:94–109
Wootton RJ (1990) Ecology of teleost fishes. Chapman and Hall, London
Zhu SQ, Oberdorster E, Haasch ML (2006) Toxicity of an engineered nanoparticle (fullerene, C60) in two aquatic species, Daphnia and fathead minnow. Mar Environ Res 62:S5–S9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Chatterjee, N., Bhattacharjee, B. & Lu, CH. Hazardous effect of ZnS nanoparticles on the feeding behaviour, growth and maturation process of the Asian striped catfish, Mystus vittatus (Bloch, 1794). Int Aquat Res 6, 113–125 (2014). https://doi.org/10.1007/s40071-014-0071-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40071-014-0071-9