Abstract
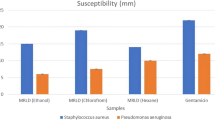
Antibiotic resistance remains one of the global challenges that needs urgent attention, including finding alternative treatment options to conventional antibiotics and reversing resistance. Pinus sylvestris L. (Pinaceae) essential oil (EO) has several biological properties. In this study, the EO of P. sylvestris was extracted by hydro-distillation method, and twenty-six chemical constituents of the EO identified by gas chromatography-mass spectrophotometry were screened as potential inhibitors of β-lactamase in silico by molecular docking. Antibacterial activity of the EO against thirteen bacterial isolates was further evaluated. Terpineol was identified as the major phytoconstituent of the P. sylvestris needles EO. From the docking analysis, benzene, 1,3-bis(3-phenoxyphenoxy) and dichloroacetic acid, 1-adamantylmethyl ester showed good inhibitory potentials against AmpC and OXA-23 β-lactamase, respectively, binding to the important catalytic serine residues (Ser64 and Ser79, respectively) with lower binding affinity energies (benzene, 1,3-bis(3-phenoxyphenoxy) = −10.1 kcal/mol and dichloroacetic acid, 1-adamantylmethyl ester = −8.4 kcal/mol) compared to the FDA-approved β-lactamase inhibitor, avibactam (−6.5 and −6.6 kcal/mol for AmpC and OXA23 β-lactamase, respectively). The EO inhibited the growth of all the tested bacteria. Klebsiella pneumoniae and Micrococcus luteus were the most susceptible bacteria with an inhibition zone (ZI) of 24 mm each, while the ZI obtained for Proteus vulgaris was the lowest (ZI = 8 mm). The results obtained in this study showed that EO of P. sylvestris has potential as a treatment option for broad-spectrum bacterial infections and in the management of β-lactamase-related antibiotic resistance.





Similar content being viewed by others
References
Öztürk H, Ozkirimli E, Özgür A (2015) Classification of beta-lactamases and penicillin binding proteins using ligand-centric network models. PLoS ONE 10(2):e0117874. https://doi.org/10.1371/journal.pone.0117874
Munita JM, Arias CA (2016) Mechanisms of antibiotic resistance. Microbiol Spectr 4(2):4–2. https://doi.org/10.1128/microbiolspec.VMBF-0016-2015
Coutinho HDM, Matias EFF, Santos KKA, Tintino SR, Souza CES, Guedes GMM, Santos FAD, Costa JGM, Falcão-SilvaIqueira-Júnior VSJP (2010) Enhancement of the norfloxacin antibiotic activity by gaseous contact with the essential oil of Croton zehntneri. J Young Pharm 2(4):362–364. https://doi.org/10.4103/0975-1483.71625
Almeida RS, Freitas PR, Araújo ACJ, Alencar Menezes IR, Santos EL, Tintino SR, Moura TF, Ferreira VA, Silva ACA, Silva LE, do Amaral W, (2020) GC-MS profile and enhancement of antibiotic activity by the essential oil of Ocotea odorifera and safrole: Inhibition of Staphylococcus aureus efflux pumps. Antibiotics 9(5):247. https://doi.org/10.3390/antibiotics9050247
Jin J, Zhang JY, Guo N, Sheng H, Li L, Liang JC, Wang XL, Li Y, Liu MY, Wu XP, Yu L (2010) Farnesol, a potential efflux pump inhibitor in Mycobacterium smegmatis. Molecules 15(11):7750–7762. https://doi.org/10.3390/molecules15117750
Veras HN, Rodrigues FF, Botelho MA, Menezes IR, Coutinho HD, Costa JG (2017) Enhancement of aminoglycosides and β-lactams antibiotic activity by essential oil of Lippia sidoides Cham. and the thymol. Arab J Chem 10:S2790–S2795. https://doi.org/10.1016/j.arabjc.2013.10.030
Dhara L, Tripathi A (2013) Antimicrobial activity of eugenol and cinnamaldehyde against extended spectrum beta lactamase producing enterobacteriaceae by in vitro and molecular docking analysis. Eur J Integr Med 5(6):527–536. https://doi.org/10.1016/j.eujim.2013.08.005
Fayemiwo KA, Adeleke MA, Okoro OP, Awojide SH, Awoniyi IO (2014) Larvicidal efficacies and chemical composition of essential oils of Pinus sylvestris and Syzygium aromaticum against mosquitoes. Asian Pac J Trop Biomed 4(1):30–34. https://doi.org/10.1016/S2221-1691(14)60204-5
Kızılarslan Ç, Sevg E (2013) Ethnobotanical uses of genus Pinus L. (Pinaceae) in Turkey. Indian J Tradit Knowl 12(2):209–220. http://nopr.niscair.res.in/handle/123456789/16860
Sonibare OO, Olakunle K (2008) Chemical composition and antibacterial activity of the essential oil of Pinus caribaea from Nigeria. Afr J Biotechnol 7(14):2462–2464
Lin X, Li X, Lin X (2020) A review on applications of computational methods in drug screening and design. Molecules 25(6):1375. https://doi.org/10.3390/molecules25061375
Elyemni M, Louaste B, Nechad I, Elkamli T, Bouia A, Taleb M, Chaouch M, Eloutassi N (2019) Extraction of essential oils of Rosmarinus officinalis L. by two different methods: Hydrodistillation and microwave assisted hydrodistillation. Sci World J 2019: 6. https://doi.org/10.1155/2019/3659432
Adams RP (2007) Identification of essential oil components by gas chromatography/ mass spectrometry, 4th edn. Allured Publ, Carol Stream, IL
Palzkill T (2018) Structural and mechanistic basis for extended-spectrum drug-resistance mutations in altering the specificity of TEM, CTX-M, and KPC β-lactamases. Front Mol Biosci 5:16. https://doi.org/10.3389/fmolb.2018.00016
Goldberg SD, Iannuccilli W, Nguyen T, Ju J, Cornish VW (2003) Identification of residues critical for catalysis in a class C β-lactamase by combinatorial scanning mutagenesis. Protein Sci 12(8):1633–1645. https://doi.org/10.1110/ps.0302903
Smith CA, Antunes NT, Stewart NK, Toth M, Kumarasiri M, Chang M, Mobashery S, Vakulenko SB (2013) Structural basis for carbapenemase activity of the OXA-23 β-lactamase from Acinetobacter baumannii. Chem Biol 20(9):1107–1115. https://doi.org/10.1016/j.chembiol.2013.07.015
Dallakyan S, Olso AJ (2015) Small-molecule library screening by docking with PyRx. In: Hempel J, Williams C, Hong C (eds) Chemical biology. methods in molecular biology, vol 1263. Humana Press, New York, NY pp 243–250. https://doi.org/10.1007/978-1-4939-2269-7_19
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7(1):1–13. https://doi.org/10.1038/srep42717
Banerjee P, Eckert AO, Schrey AK, Preissner R (2018) ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res 46(W1):W257–W263. https://doi.org/10.1093/nar/gky318
Akinpelu DA, Abioye EO, Aiyegoro OA, Akinpelu OF, Okoh AI (2015). Evaluation of antibacterial and antifungal properties of Alchornea laxiflora (Benth.) Pax. & Hoffman. Evid Based Complement Altern 2015. https://doi.org/10.1155/2015/684839
Szmigielski R, Cieslak M, Rudziński KJ, Maciejewska B (2012) Identification of volatiles from Pinus silvestris attractive for Monochamus galloprovincialis using a SPME-GC/MS platform. Environ Sci Pollut Res 19(7):2860–2869. https://doi.org/10.1007/s11356-012-0792-5
Badawy ME, Marei GIK, Rabea EI, Taktak NE (2019) Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pestic Biochem Physiol 158:185–200. https://doi.org/10.1016/j.pestbp.2019.05.008
Turchetti G, Garzoli S, Laghezza Masci V, Sabia C, Iseppi R, Giacomello P, Tiezzi A, Ovidi E (2020) Antimicrobial testing of Schinus molle (L.) leaf extracts and fractions followed by GC-MS investigation of biological active fractions. Molecules 25(8):1977. https://doi.org/10.3390/molecules25081977
Meng XY, Zhang HX, Mezei M, Cui M (2011) Molecular docking: a powerful approach for structure-based drug discovery. Curr Comput-Aided Drug Des 7(2):146–157. https://doi.org/10.2174/157340911795677602
González-Bello C, Rodríguez D, Pernas M, Rodríguez Á, Colchón E (2020) β-Lactamase inhibitors to restore the efficacy of antibiotics against superbugs. J Med Chem 63(5):1859–1881. https://doi.org/10.1021/acs.jmedchem.9b01279
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23(1–3):3–25. https://doi.org/10.1016/S0169-409X(96)00423-1
Morak-Młodawska B, Jeleń M, Pluta K (2021) Phenothiazines modified with the pyridine ring as promising anticancer agents. Life 11(3):206. https://doi.org/10.3390/life11030206
Carrière F (2016) Impact of gastrointestinal lipolysis on oral lipid-based formulations and bioavailability of lipophilic drugs. Biochimie 125:297–305. https://doi.org/10.1016/j.biochi.2015.11.016
Salamon I, Kryvtsova M, Bucko D, Tarawneh, AH (2021). Chemical characterization and antimicrobial activity of some essential oils after their industrial large-scale distillation. J Microbiol Biotechnol Food Sci 2021: 984–988. https://doi.org/10.15414/jmbfs.2018.8.3.965-969.
Njoku IS, Rahman NU, Khan AM, Otunomo I, Asekun OT, Familoni OB, Ngozi C (2022) Chemical composition, antioxidant, and antibacterial activity of the essential oil from the leaves of Pinus sylvestris. Pac J Sci Technol 23(1):85–93
Mitić ZS, Jovanović B, Jovanović SČ, Mihajilov-Krstev T, Stojanović-Radić ZZ, Cvetković VJ, Mitrović, TL, Marin PD, Zlatković BK, Stojanović GS (2018) Comparative study of the essential oils of four Pinus species: Chemical composition, antimicrobial and insect larvicidal activity. Ind Crops Prod 111(2018):55–62
Acknowledgements
The authors appreciate the help of technologists at the Microbiology and Chemistry Department of Osun State University during the laboratory investigation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement: The study showed that P. sylvestris essential oil has broad-spectrum antibacterial activity and its phytoconstituents identified by GC-MS have the potential to inhibit β-lactamase enzymes.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oyewole, K.A., Oyedara, O.O., Awojide, S.H. et al. Antibacterial and In Silico Evaluation of β-lactamase Inhibitory Potential of Pinus sylvestris L. (Scots Pine) Essential Oil. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 93, 967–977 (2023). https://doi.org/10.1007/s40011-023-01490-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-023-01490-3