Abstract
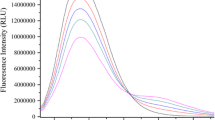
Three new p-halogenated cinnamic amides (p-CA) have been synthesized and the interactions between p-CA and bovine serum albumin (BSA) in pH 7.4 tris–HCl buffer solution have been investigated by fluorescence and ultraviolet spectroscopies. Fluorescence spectra revealed that the quenching was a static quenching process at low p-CA concentrations and a combined quenching (static and dynamic) process at higher p-CA concentrations. The Stern–Volmer quenching constants (K SV), the binding constants (K A), the number of binding sites (n), and the thermodynamic parameters (ΔG, ΔH, and ΔS) have been calculated at 298, 303, and 308 K. According to Föster’s non-radiative energy transfer theory, the binding distances between p-CA and BSA are determined and the resonance energy transfer in p-CA and BSA has high possibility. The investigation of synchronous fluorescence spectra suggested that the binding of p-CA to BSA changed the microenvironment around the tyrosine residues. In addition, three halogens have different effects on binding interactions when they are introduced to the cinnamic amides.








Similar content being viewed by others
References
Khodarahmi R, Karimi SA, Kooshk MRA, Ghadami SA, Ghobadi S, Amani M (2012) Comparative spectroscopic studies on drug binding characteristics and protein surface hydrophobicity of native and modified forms of bovine serum albumin: possible relevance to change in protein structure/function upon non-enzymatic glycation. Spectrochim Acta A 89:177–186
He XM, Carter DC (1992) Atomic structure and chemistry of human serum albumin. Nature (London) 358:209–215
Madureira AR, Pereira CI, Gomes AMP, Pintado ME, Malcata FX (2007) Bovine whey proteins—overview on their main biological properties. Food Res Int 40:1197–1211
Samanta A, Paul BK, Guchhait N (2011) Spectroscopic probe analysis for exploring probe-protein interaction: a mapping of native, unfolding and refolding of protein bovine serum albumin by extrinsic fluorescence probe. Biophys Chem 156:128–139
Tramontano A (2005) The ten most wanted solutions in protein bioinformatics. CRC Press, London
Arun N, Subramanian P, Boobathy S (2011) Antibacterial activity screening and HPLC analysis of crude extract from Diacure a polyherbal formulation. Elixir Online J 36:3110–3113
Huang L, Lin C, Li A, Wei B, Teng J, Li L (2010) Pro-coagulant activity of phenolic acids isolated from Blumea riparia. Nat Prod Commun 5:1263–1266
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, New York
Schmid FX (1989) Spectral methods of characterizing protein conformation and conformational changes. Protein structure: A Practical Approach. IRL Press, Oxford
Meng F, Zhu J, Zhao A, Yu S, Lin C (2012) Synthesis of p-hydroxycinnamic acid derivatives and investigation of fluorescence binding with bovine serum albumin. J Lumin 132:1290–1298
Lin H, Lan J, Guan M, Sheng F, Zhang H (2009) Spectroscopic investigation of interaction between mangiferin and bovine serum albumin. Spectrochim Acta A 73A:936–941
Song S, Hou X, Wu Y, Shuang S, Yang C, Inoue Y, Dong C (2009) Study on the interaction between methyl blue and human serum albumin by fluorescence spectrometry. J Lumin 129:169–175
Ware WR (1962) Oxygen quenching of fluorescence in solution: an experimental study of the diffusion process. J Phys Chem 66:455–458
Anbazhagan V, Renganathan R (2008) Study on the binding of 2,3-diazabicyclo[2.2.2]oct-2-ene with bovine serum albumin by fluorescence spectroscopy. J Lumin 128(9):1454–1458
Olsson TS, Williams MA, Pitt WR, Ladbury JE (2008) The thermodynamics of protein-ligand interaction and solvation: insights for ligand design. J Mol Biol 384(4):1002–1017
Hammes GG (2000) Thermodynamics and kinetics for the biological sciences. John Wiley, New York
Homans SW (2007) Dynamics and thermodynamics of ligand-protein interactions. Top Curr Chem 272:51–82
Ross PD, Subramanian S (1981) Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20:3096–3102
Paul BK, Guchhait N (2011) A spectral deciphering of the binding interaction of an intramolecular charge transfer fluorescence probe with a cationic protein: thermodynamic analysis of the binding phenomenon combined with blind docking study. Photochem Photobiol Sci 10:980–991
Hammes GG (2005) Spectroscopy for the biological sciences. John Wiley, Hoboken
Cyril L, Earl JK, Sperry WM (1961) Biochemists’ Handbook. Epon Led Press, London
Lloyd JBF (1971) Synchronized excitation of fluorescence emission spectra. Nature Phys Sci (London) 231:64–65
Miller JN (1984) Recent developments in fluorescence and chemiluminescence analysis. Analyst (London) 109:191–198
Kuznetsova IM, Yakusheva TA, Turoverov KK (1999) Contribution of separate tryptophan residues to intrinsic fluorescence of actin. Analysis of 3D structure. FEBS Lett 452:205–210
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (21362001), Natural Science Foundation of Guangxi Province (2013GXNSFDA019005), Youth Foundation of Guangxi Botanical Garden of Medicinal Plant project (Grant No. guiyaoji201108), and the Guangxi University Foundation of Training Undergraduate Students’ Experimental Skills and Innovational Abilities (SYJN20120306).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, J., He, Y., Bei, G. et al. Fluorometric Investigation of the Interaction of (2E)-3-(4′-Halophenyl)-N-{4′′-[(5′′′,6′′′-dimethoxypyrimidin-4′′′-yl)sulfamoyl]phenyl}prop-2-enamides with Bovine Serum Albumin. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 84, 505–516 (2014). https://doi.org/10.1007/s40010-014-0165-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-014-0165-1