Abstract
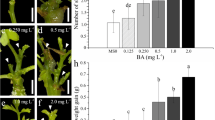
Morphogenetic potential of cotyledon and hypocotyl explants of cabbage (Brassica oleracea L. var. capitata) was studied to develop a reliable plant regeneration protocol. Nine days old aseptically grown seedlings of cabbage were used as source of explant for plant regeneration. Out of 36 combinations of growth regulators used in MS medium, high frequency shoot regeneration from cotyledon explant was obtained on MS medium supplemented with 1.5 mg/l BAP and 0.50 mg/l NAA and 2 mg/l BAP and 0.25 mg/l IAA respectively. High percentage root regeneration (90 %) in in vitro developed shoots were obtained on MS medium supplemented with 0.10 mg/l IBA. The regenerated complete plantlets were transferred to pots containing a mixture of sand and soil and acclimatized. A reproducible and efficient plant regeneration protocol has been standardized in cabbage cv. Pride of India.

Similar content being viewed by others
Abbreviations
- BAP:
-
N 6-Benzylaminopurine
- NAA:
-
Naphthalene acetic acid
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog
References
Munger HM (1988) Adaptation and breeding of vegetable crops for improved human nutrition. In: Quebedeaux B, Pedrick AB (eds) Horticulture and human health
Zhang Y, Talaly P, Cho CG, Posner GH (1992) A major inducer of anticarcinogenic protective enzymes from broccoli isolation and elucidation of structure. Proc Natl Acad Sci USA 89:2399–2403
Deng-Xia, Lei C, Yu-Mei L, Mu Z, Yang-Yong Z, Zhi-Yuan F, Li-Mei Y (2011) Transformation of cabbage (Brassica oleracea L. var.capitata) with Bt cry1Ba3 gene for control of diamondback moth. Agric Sci China 10(11):1693–1700
Fan MQ, Zhu YL, Zhu LH (2005) Studies on highly efficient in vitro shoot regeneration from cotyledon of Chinese cabbage. J Nanjing Agric Univ 28(1):20–23
Zhao J, Liang A, Zhu Z, Tang Y (2006) Regeneration of Chinese transgenic plants expressing antibacterial peptide gene and cowpea trypsin inhibitor gene. Euphytica 150:397–406
Memon SA, Hou Xilin, Zhu Bo, Wolukau Joseph N (2008) High-frequency adventitious shoots regeneration from leaf of non-heading Chinese cabbage (Brassica campestris ssp.chinensis) cultured in vitro. Acta Physiol Plant 31:1191–1196
Xue-Yun Z, Shi-Jie C, Li-Ping C, Ming-Fang Z, Jing-Quan Y (2010) Induction and origin of adventitious roots from chimeras of Brassica juncea and Brassica oleracea. Plant Cell Tissue Organ Cult 101:287–294
Cao J, Anthony MS, Elizabeth DE (2008) Sequential transformation to pyramid two Bt genes in Brassica juncea L. and its potential for control of diamondback moth larvae. Plant Cell Rep 27(3):479–487
Yu ZD, He QW, Mu JH, Kang J, Wang PW (2005) Study on the tissue culture and plant regeneration of Chinese cabbage. J Jitin Agric Univ 27(4):391–395
Chen LP, Zhang MF, Xiao QB, Wu JG, Hirata Y (2004) Plant regeneration from hypocotyls protoplasts of red cabbage (Brassica oleracea) by using nurse cultures. Plant Cell Tissue Organ Cult 77(2):133–138
Kirti PB, Bhat SR, Kumar VD, Prakash S, Chopra VL (2001) A simple protoplast for regenerating mesophyll protoplasts of vegetable brassicas. J Plant Biochem Biotechnol 10(1):49–51
Ferrie AMR, Mollers C (2010) Haploids and doubled haploids in Brassica species for genetic and genomic research. Plant Cell Tissue Organ Cult 14:531–541
Murashsige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479
Srivastava DK, Andrianova VM, Piruzian ES (1991) Regeneration and genetic transformation studies in watermelon (Citrullus vulgaris L. cv. Melitopolski). In: Prakash J, Pierik R L M, (Eds) Horticulture—new technologies and applications, pp 127–130
Srivastava V, Reddy AS, Guha Mukherjee S (1988) Transformation and regeneration of Brassica oleracea mediated by an oncogenic Agrobacterium tumefaciens. Plant Cell Rep 7:504–507
Srivastava DK, Andrianov VM, Priuzian ES (1989) Tissue culture and plant regeneration of watermelon (Citrullus vulgaris schrad cv. Meletopolski). Plant Cell Rep 8:300–302
Srivastava DK, Kolgonova TV, Mett VL, Piruzian ES (1991) Genetic transformation of cotton (Gossypium hirsutum L. Cv. 108-F). Acta Hortic 289:263–264
Baroda A, Madanpotra S, Singh ND and Jaiwal PK (2003) Stable transformation of Brassica juncea using Agrobacterium tumefaciens. In: Proceedings of 2nd international congress of plant physiology, Abstract no. S11-P8, p 462
Chakrabarty R, Viswakarma N, Bhat SR, Kirti PB, Singh BD, Chopra VL (2002) Agrobacterium mediated transformation of cauliflower: optimization of protocol and development of Bt-transgenic cauliflower. J Biosci 27(25):495–502
Dixit S, Srivastava DK, Sharma DR (1998) Genetic transformation in cauliflower (Brassica oleracea L. var. botrytis cv. Pusa Snow Ball). In: Proceedings of national symposium on biotechnology in agriculture and environment, Abstract p 2
Sharma S, Srivastava DK, Nath AK, Sharma DK (1996) Regeneration and genetic transformation studies in cauliflower (Brassica oleracea L. var. botrytis cv. Pusa Snow Ball). In: Proceedings of national symposium on horticulture biotechnology, Abstract No. V-120
Dong JZ, Jia SR (1991) High efficiency plant regeneration from cotyledons of watermelon (Citrullus vulgaris Schard). Plant Cell Rep 9:858–863
Guo DP, Zhu ZJ, Hu XX, Zheng SJ (2005) Effect of cytokinins on shoot regeneration from cotyledon and leaf segment of stem mustard (Brassica juncea var. tsatsai). Plant Cell Tissue Organ Cult 83:123–127
Arora N, Yadav NR, Yadav RC, Chowdhury JB (1997) Role of IAA and BAP on plant regeneration in cultured cotyledons of cauliflower. Crucif Newslett 19:41–42
Rafat A, Aziz MA, Rhashid AA, Abdullah SNA, Kamaladini H, Sirchi MHT, Javadi MB (2010) Optimization of Agrobacterium tumefaciens-mediated transformation and shoot regeneration after co-cultivation of cabbage (Brassica oleracea subsp. capitata) cv. KY cross with At HSP101 gene. Sci Hortic 124:1–8
Jin RG, Liu YB, Tabashnik BE, Borthakur D (2000) Development of transgenic cabbage (Brassica oleracea var. capitata) for insect resistance by Agrobacterium tumefaciens-mediated transformation. In Vitro Cell Dev Biol Plant 36:231–237
Yang JL, Seong ES, Kim MJ, Ghimire BK, Kang WH, Chang YY, Cheng HL (2010) Direct somatic embryogenesis from pericycle cell of broccoli (Brassica oleracea L. var. italica) root explants. Plant Cell Tissue Organ Cult 100:49–58
Bhalla PL, Weerd ND (1999) In vitro propagation of cauliflower, Brassica oleracea var. botrytis for hybrid seed production. Plant Cell Tissue Organ Cult 56:89–95
Sretenovic RT, Ninkovic S, Vinterhalter B, Milijus DJ, Vinterhalter D (2004) Introduction of resistance to herbicide Basta Reg. in Savoy cabbage. Biol Plant 48(3):431–436
Dai SY, Jai S, Dong G, Merg LY (1994) In vitro culture and plant regeneration of cotyledon and hypocotyls of cabbage. Adv Hortic 12:286–289
Eisner GI, Mar Yakhina I, Shenyakin MF (1992) Optimization of conditions for in vitro plant regeneration for cabbage transformations. Sov Agric Sci 11(12):15–19
Petrova S, Antonova G (1996) Plant regeneration from seedling explants of cabbage. Crucif Newslett 18:31
Zhang S, Wen FJ, Wei YT, Fu LH (1997) Optimization of growth regulators in medium for efficient plant regeneration of Chinese cabbage. Crucif Newslett 19:51–52
Cao MQ, Charlot F, Dore C (1990) Embryogenesis and plant regeneration in sauerkraut cabbage (Brassica oleracea L. subsp. capitata) by in vitro culture of isolated microspores. Comptes Rendud de l’Academie des Science 310(5):203–209
Lillo C, Shahin EA (1986) Rapid regeneration of plants from hypocotyl protoplasts and root segments of cabbage. HortScience 21(3):315–317
Koda T, Ichi T, Yamagishi H, Yoshikawa H (1988) Effect of phytohormones and gelling agents on plant regeneration from protoplasts of red cabbage. Agric Biol Chem 52(9):2337–2340
Hegazi HH, Matsubara S (1991) Plant regeneration from hypocotyl callus of inter-generic and interspecific hybrids between radish (Raphanus sativus L. daikon and radicula gropus), cabbage (Brassica oleracea L. capitata group) and Chinese cabbage (Brassica campestris L. pekinsis group). J Jpn Soc Hortic Sci 60(2):369–377
Acknowledgments
Authors thank to Professor and Head, Dept. of Vegetable Science of Dr. Y.S. Parmar University of Horticulture and Forestry, Solan, India for providing germplasm of cabbage cv. Pride of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, S., Gambhir, G. & Srivastava, D.K. High Frequency Organogenesis in Cotyledon and Hypocotyl Explants of Cabbage (Brassica oleracea L. var. capitata). Natl. Acad. Sci. Lett. 37, 5–12 (2014). https://doi.org/10.1007/s40009-013-0191-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40009-013-0191-6