Abstract
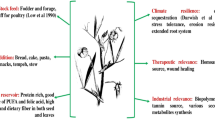
Schleichera oleosa (Lour.) Oken (a monotypic genus), belonging to family Sapindaceae, has been reported for its medicinal importance as well as its extensive use for lac cultivation in the Indo-Chinese subcontinent. Lac, secreted by females of lac insect Kerria lacca (Kerr), has tremendous industrial applications. Thirty-seven good, bad and intermediate lac yielding trees of S. oleosa were selected for molecular characterization using RAPD markers. Twenty-one random decamer primers revealed 81.4 % average polymorphism among selected trees. The Jaccard’s similarity coefficient value was 0.617, indicating very high genetic diversity between these trees. No strong grouping was observed among good and bad lac yielders while most of the intermediate lac yielders grouped together in UPGMA and PCA analysis. Genetic diversity indices were lowest in intermediate lac yielders while comparable in good and bad lac yielders. The low genetic differentiation (Fst) and gene flow (Nm) was estimated among good and bad yielding populations. On the other hand, high genetic differentiation and restricted gene flow was estimated among good and intermediate as well as among bad and intermediate yielder populations. The unique DNA profile of each trees suggests that trees of S. oleosa under the use of lac cultivation were seed propagated rather than via any vegetative mode of propagation. This study would also be helpful in future for their true to type multiplication through tissue culture and breeding programs for elite trees.



Similar content being viewed by others
References
Mabberley DJ (1997) The plant-book: a portable dictionary of the vascular plants. Cambridge University Press, UK
Bhatia H, Kaur J, Nandi S, Gurnani V, Chaudhary A, Reddy PH, Vashishtha A, Rathi B (2012) A Review on Schleichera oleosa: pharmacological and environmental aspects. J Pharm Res. doi:10.1016/j.jopr.2012.11.003
Rao AR, Singh P (1990) Lac cultivation and marketing. Indian For 116(5):459–463
Sharma KK, Ramani R (1999) An update on synoptic catalogue of lac insects (Homoptera: Tachardidae). J Bombay Nat Hist Soc 96:438–443
Iwasa S (1997) Schleichera oleosa (Lour.) Oken. In: Faridah Hanum I, Van der Maesen LJG (eds) Plant resources of south-east Asia No. 11. Auxiliary plants. Prosea Foundation, Bogor, pp 227–229
Clegg MT (1989) Molecular diversity in plant populations. In: Brown AHD, Clegg MT, Kahler AL, Weir BS (eds) Plant population genetics breeding and genetic resources. Sinauer, Sunderland, pp 98–115
Hamrick JL, Godt MJW, Murawski DA, Loveless MD (1991) Correlations between species traits and allozyme diversity: implications for conservation biology. In: Falk D, Holsinger K (eds) Genetics and conservation of rare plants. Oxford Press, London, pp 75–86
Storfar A (1996) Quantitative genetics: a promising approach for the assessment genetic variation in endangered species. Trends Ecol Evol 11:343–348
Song C, Bao M (2006) Genetic diversity of RAPD marker for natural Davidia involucrata populations. Front For China 1:95–99
Miller JC, Tanksley SD (1990) Effects of restriction enzymes, probe source, and probe length on detecting restriction fragment length polymorphism in tomato. Theor Appl Genet 80:385–389
Welsh J, McClelland M (1990) Fingerprinting genomes using PCR with arbitrary primers. Nucl Acid Res 18:7213–7218
Williams JG, Kubelik AR, Livak J, Rafalski JA, Tingey SV (1990) DNA polymorphism amplified by arbitrary primers are useful as genetic markers. Nucl Acid Res 18:6531–6535
Xue X, Wang Y, Korpelainen H, Li C (2007) Genetic diversity of Picea asperata populations based on RAPDs. Plant Biol 9:101–108
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location and population dynamics. Proc Natl Acad Sci 81:8014–8018
Sneath PHA, Sokal RR (1973) Numerical taxonomy: the principles and practice of numerical classification. W. H. Freeman, San Francisco, p 573
Rohlf FJ (1992) NTSYS-pc. Numerical Taxonomy and Multivariate Analysis System, Version 1.7 Exeter Software, New York
Yeh FC, Yang RC, Boyle T (1999) POPGENE 32-version 1.32. Population Genetics Software. http.//www.ualberta.ca/~fyeh/fyeh/. Accessed Dec 2008
Wright S (1951) The genetical structure of populations. Ann Eugen 15:323–354
Singh A, Negi MS, Rajagopal J, Bhatia S, Tomar UK, Srivastava PS, Lakshimikumaran M (1999) Assessment of genetic diversity in Azadirachta indica using AFLP markers. Theor Appl Genet 99:272–279
Gillies ACM, Cornelius JP, Newton AC, Navarro C, Hernández M, Wilson J (1997) Genetic variation in Costa Rican populations of the tropical timber species Cedrela odorata L., assessed using RAPDs. Mol Ecol 6:1133–1145
Heider B, Andersson MS, Schultze-Kraft R (2007) RAPD variation among North Vietnamese Flemingia macrophylla (Willd.) Kuntze ex Merr. Accessions. Biodivers Conserv 16:1617–1631
Petit RJ, Hampe A (2006) Some evolutionary consequences of being a tree. Annu Rev Ecol Evol Syst 37:187–214
Gautam M (2007) Phylogeny and floral biology of Schleichera oleosa (Lour.) Oken (Sapindaceae). M. Phil. Thesis, University of Delhi
Rands SA, Whitney HM (2011) Field margins, foraging distances and their impacts on nesting pollinator success. PLoS ONE 6(10):e25971. doi:10.1371/journal.pone.0025971
Acknowledgments
Authors thank Dr. K. V. Bhat, NBPGR, PUSA, Delhi for helpful discussions on data analysis. Financial support and encouragement from Department of Biotechnology (DBT), Govt. of India, India is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vashishtha, A., Jehan, T., Sharma, K. et al. Molecular Characterization and Genetic Diversity of Schleichera oleosa (Lour.) Oken: Major Host Tree for Lac Cultivation. Natl. Acad. Sci. Lett. 36, 429–435 (2013). https://doi.org/10.1007/s40009-013-0152-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40009-013-0152-0