Abstract
Ocular drug delivery has been widely recognized as an attractive route for drug administration for cures of ocular diseases. Overall, designing an effective therapy to cure ocular diseases has been considered as a formidable task. Even though some infectious or inflammatory eye diseases could be alleviated by eye drops or ointments, achieving the required therapeutic efficacy along with ocular bioavailability remains a challenge due to the numerous anatomical and physiological barriers prevailing in the eye. Drug delivery to the posterior segment of the eye is yet a more challenging task. In this context, a better understanding of physiologic natures of the eyes and ocular pharmacokinetics would facilitate the development of new drug delivery systems to treat various vision-threatening disorders. For the effective drug delivery to target sites and to enhance the ocular bioavailability, recent progress in formulation strategies using nanotechnologies holds promises in terms of devising improved ophthalmic medicines. Hence, this review presents an overview of various aspects of ocular drug delivery, with a specific emphasis on nanocarrier-based strategies, including physiological barriers in eyes and conventional drug formulations. Recent research on sustained, controlled, and targeted systems for ocular drug delivery was updated as well.

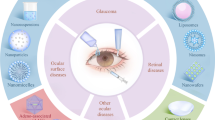
[Reproduced with modifications from Liu et al. (2012), with permission]
Similar content being viewed by others
References
Abd El-Gawad AEH, Soliman OA, El-Dahan MS, Al-Zuhairy SAS (2017) Improvement of the ocular bioavailability of econazole nitrate upon complexation with cyclodextrins. AAPS Pharm Sci Tech 18:1795–1809
Abdelbary GA, Amin MM, Zakaria MY (2017) Ocular ketoconazole-loaded proniosomal gels: formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv 24:309–319
Achouri D, Alhanout K, Piccerelle P, Andrieu V (2013) Recent advances in ocular drug delivery. Drug Dev Ind Pharm 39:1599–1617
Agnihotri SM, Vavia PR (2009) Diclofenac-loaded biopolymeric nanosuspensions for ophthalmic application. Nanomed Nanotechnol Biol Med 5:90–95
Agueros M, Zabaleta V, Espuelas S, Campanero MA, Irache JM (2010) Increased oral bioavailability of paclitaxel by its encapsulatin through complex formation with cyclodextrins in poly(anhydride) nanoparticles. J Control Release 145:2–8
Akula SK, Ma PE, Peyman GA, Rahimy MH, Hyslop NE, Janney A, Ashton P (1994) Treatment of cytomegalovirus retinitis with intravitreal injection of liposome encapsulated ganciclovir in a patient with AIDS. Br J Ophthalmol 78:677–680
Al-Halafi AM (2014) Nanocarriers of nanotechnology in retinal diseases. Saudi J Ophthalmol 28:304–309
Ali HS, York P, Ali AM, Blagden N (2011) Hydrocortisone nanosuspensions for ophthalmic delivery: a comparative study between microfluidic nanoprecipitation and wet milling. J Control Release 149:175–181
Allam AN, El Gamal S, Naggar V (2011) Formulation and evaluation of acyclovir niosomes for ophthalmic use. Asian J Pharm Biol Res 1:28–40
Allen TM, Cullis PR (2013) Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 65:36–48
Ambati J, Adamis AP (2002) Transscleral drug delivery to the retina and choroid. Prog Retin Eye Res 21:145–151
Ames P, Galor A (2015) Cyclosporine ophthalmic emulsions for the treatment of dry eye: a review of the clinical evidence. Clin Investig (Lond) 5:267–285
Anand S, Braga VML (2016) Cyclodextrins in ocular drug delivery. In: Nano-biomaterials for ophthalmic drug delivery. Springer, Cham, pp 243–252
Anand BS, Mitra AK (2002) Mechanism of corneal permeation of L-valyl ester of acyclovir: targeting the oligopeptide transporter on the rabbit cornea. Pharm Res 19:1194–1202
Baranowski P, Karolewicz B, Gajda M, Pluta J (2014) Ophthalmic drug dosage forms: characterisation and research methods. Sci World J 2014:861904
Barar J, Aghanejad A, Fathi M, Omidi Y (2016) Advanced drug delivery and targeting technologies for the ocular diseases. Bioimpacts 6:49–67
Barot RK, Shitole SC, Bhagat N, Patil D, Sawant P, Patil K (2016) Therapeutic effect of 0.1% tacrolimus eye ointment in allergic ocular diseases. J Clin Diagn Res 10:NC05–N09
Bourlais CL, Acar L, Zia H, Sado PA, Needham T, Leverge R (1998) Ophthalmic drug delivery systems—recent advances. Prog Retin Eye Res 17:33–58
Calles JA, Lopez-Garcia A, Valles EM, Palma SD, Diebold Y (2016) Preliminary characterization of dexamethasone-loaded cross-linked hyaluronic acid films for topical ocular therapy. Int J Pharm 509:237–243
Chou TY, Hong BY (2014) Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: background, effectiveness, tolerability, safety, and future applications. Ther Clin Risk Manag 10:665–681
Comstock TL, Paterno MR, Singh A, Erb T, Davis E (2011) Safety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgery. Clin Ophthalmol 5:177–186
Conway BR (2008) Recent patents on ocular drug delivery systems. Recent Patents Drug Deliv Formulation 2:1–8
Davis BM, Normando EM, Guo L, Turner LA, Nizari S, O’Shea P, Moss SE, Somavarapu S, Cordeiro MF (2014) Topical delivery of Avastin to the posterior segment of the eye in vivo using annexin A5-associated liposomes. Small 10:1575–1584
Dey S, Mitra AK (2005) Transporters and receptors in ocular drug delivery: opportunities and challenges. Expert Opin Drug Deliv 2:201–204
Ding S (1998) Recent developments in ophthalmic drug delivery. Pharm Sci Technol Today 1:328–335
Drew WL, Ives D, Lalezari JP, Crumpacker C, Follansbee SE, Spector SA, Benson CA, Friedberg DN, Hubbard L, Stempien MJ (1995) Oral ganciclovir as maintenance treatment for cytomegalovirus retinitis in patients with AIDS. N Engl J Med 333:615–620
du Toit LC, Pillay V, Choonara YE, Govender T, Carmichael T (2011) Ocular drug delivery—a look towards nanobioadhesives. Expert Opin Drug Deliv 8:71–94
Dubey A, Prabhakara P (2014) Ocular drug delivery systems for treatment of glaucoma. Int J Pharm Sci Nanotech 7:1–11
Dugel PU, Bandello F, Loewenstein A (2015) Dexamethasone intravitreal implant in the treatment of diabetic macular edema. Clin Ophthalmol 9:1321–1335
Fathi M, Barar J, Aghanejad A, Omidi Y (2015) Hydrogels for ocular drug delivery and tissue engineering. Bioimpacts 5:159–164
Freedman KA, Klein JW, Crosson CE (1993) Beta-cyclodextrins enhance bioavailability of pilocarpine. Curr Eye Res 12:641–647
Gaucher G, Dufresne M-H, Sant VP, Kang N, Maysinger D, Leroux J-C (2005) Block copolymer micelles: preparation, characterization and application in drug delivery. J Controll Release 109:169–188
Gaudana R, Jwala J, Boddu SH, Mitra AK (2009) Recent perspectives in ocular drug delivery. Pharm Res 26:1197–1216
Gaudana R, Ananthula HK, Parenky A, Mitra AK (2010) Ocular drug delivery. AAPS J 12:348–360
Geetha G, Poojitha U, Khan UAA (2014) Various techniques for preparation of nanosuspension—a review. Int J Pharma Res Rev 3:30–37
Geroski DH, Edelhauser HF (2000) Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci 41:961–964
Glogowski S, Lowe E, Siou-Mermet R, Ong T, Richardson M (2014) Prolonged exposure to loteprednol etabonate in human tear fluid and rabbit ocular tissues following topical ocular administration of Lotemax gel, 0.5%. J Ocul Pharmacol Ther 30:66–73
Gunda S, Hariharan S, Mandava N, Mitra AK (2008) Barriers in ocular drug delivery. In: Ocular transporters in ophthalmic diseases and drug delivery. Humana Press, New York, pp 399–413
Habib FS, Fouad EA, Abdel-Rhaman MS, Fathalla D (2010) Liposomes as an ocular delivery system of fluconazole: in-vitro studies. Acta ophthalmologica 88:901–904
Hathout RM, Mansour S, Mortada ND, Guinedi AS (2007) Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS Pharm Sci Tech 8:E1–E12
Hironaka K, Inokuchi Y, Tozuka Y, Shimazawa M, Hara H, Takeuchi H (2009) Design and evaluation of a liposomal delivery system targeting the posterior segment of the eye. J Control Release 136:247–253
Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: progress and challenges. Polymer 49:1993–2007
Huang AJ, Tseng SC, Kenyon KR (1989) Paracellular permeability of corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci 30:684–689
Jervis L (2017) A summary of recent advances in ocular inserts and implants. J Bioequiv Available 9:320–323
Johannsdottir S, Jansook P, Stefansson E, Loftsson T (2015) Development of a cyclodextrin-based aqueous cyclosporine A eye drop formulations. Int J Pharm 493:86–95
Jwala J (2011) Sustained release nanoparticles containing acyclovir prodrugs for ocular herpes simplex keratitis and characterization of folate transport proteins in a corneal epithelial cell line. Doctoral dissertation, University of Missouri-Kansas City
Kalam MA, Alshamsan A, Aljuffali IA, Mishra AK, Sultana Y (2016) Delivery of gatifloxacin using microemulsion as vehicle: formulation, evaluation, transcorneal permeation and aqueous humor drug determination. Drug Deliv 23:896–907
Kamaleddin MA (2017) Nano-ophthalmology: applications and considerations. Nanomedicine 13:1459–1472
Kang-Mieler JJ, Osswald CR, Mieler WF (2014) Advances in ocular drug delivery: emphasis on the posterior segment. Expert Opin Drug Deliv 11:1647–1660
Kang-Mieler JJ, Dosmar E, Liu W, Mieler WF (2017) Extended ocular drug delivery systems for the anterior and posterior segments: biomaterial options and applications. Expert Opin Drug Deliv 14:611–620
Kassem M, Rahman AA, Ghorab M, Ahmed M, Khalil R (2007) Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int J Pharm 340:126–133
Kaur IP, Smitha R (2002) Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm 28:353–369
Kaur IP, Kapil M, Smitha R, Aggarwal D (2004) Development of topically effective formulations of acetazolamide using HP-β-CD-polymer co-complexes. Curre Drug Deliv 1:65–72
Kim YC, Chiang B, Wu X, Prausnitz MR (2014) Ocular delivery of macromolecules. J Control Release 190:172–181
Kinoshita S, Awamura S, Oshiden K, Nakamichi N, Suzuki H, Yokoi N, Rebamipide Ophthalmic Suspension Phase IISG (2012) Rebamipide (OPC-12759) in the treatment of dry eye: a randomized, double-masked, multicenter, placebo-controlled phase II study. Ophthalmology 119:2471–2478
Kompella UB, Kadam RS, Lee VH (2010) Recent advances in ophthalmic drug delivery. Ther Deliv 1:435–456
Kumaran K, Karthika K, Padmapreetha J (2010) Comparative review on conventional and advanced ocular drug delivery formulations. Int J Pharm Pharm Sci 2:1–5
Lang JC (1995) Ocular drug delivery conventional ocular formulations. Adv Drug Deliv Rev 16:39–43
Lee J, Pelis RM (2016) Drug transport by the blood-aqueous humor barrier of the eye. Drug Metab Dispos 44:1675–1681
Lee SS, Hughes P, Ross AD, Robinson MR (2010) Biodegradable implants for sustained drug release in the eye. Pharm Res 27:2043–2053
Leonardi A, Van Setten G, Amrane M, Ismail D, Garrigue JS, Figueiredo FC, Baudouin C (2016) Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease; a multicenter randomized trial. Eur J Ophthalmol 26:287–296
Liu Y, Lin X, Tang X (2009) Lipid emulsions as a potential delivery system for ocular use of azithromycin. Drug Dev Ind Pharm 35:887–896
Liu S, Jones L, Gu FX (2012) Nanomaterials for ocular drug delivery. Macromol Biosci 12:608–620
Loftsson T, Stefansson E (2002) Cyclodextrins in eye drop formulations: enhanced topical delivery of corticosteroids to the eye. Acta Ophthalmol Scand 80:144–150
Loftsson T, Stefansson E (2017) Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int J Pharm 531:413–423
Loftsson T, Hreinsdottir D, Stefansson E (2007) Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: aqueous dexamethasone eye drops. J Pharm Pharmacol 59:629–635
Loftsson T, Jansook P, Stefansson E (2012) Topical drug delivery to the eye: dorzolamide. Acta Ophthalmol 90:603–608
Mahajan HS, Shah NN, Nerkar PP, Kulkarni A, Shirpur M (2012) Niosomes encapsulated with Gatiflaxacin for ocular drug delivery. Recent Adv Pharm Sci Res 1(1):28 39
Makwana SB, Patel VA, Parmar SJ (2016) Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci 6:1–6
Mannermaa E, Vellonen KS, Urtti A (2006) Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev 58:1136–1163
Maurice D, Mishima S (1984) Ocular pharmacokinetics. In: Pharmacology of the eye. Springer, Berlin, Heidelberg, pp 19–116
McConville J (2016) Special focus issue: ocular and ophthalmic drug delivery systems. Drug Dev Ind Pharm 42:513
Mishra GP, Bagui M, Tamboli V, Mitra AK (2011) Recent applications of liposomes in ophthalmic drug delivery. J Drug Deliv 2011:863734
Morrison PW, Khutoryanskiy VV (2014) Advances in ophthalmic drug delivery. Ther Deliv 5:1297–1315
Moya-Ortega MD, Alves TF, Alvarez-Lorenzo C, Concheiro A, Stefansson E, Thorsteinsdottir M, Loftsson T (2013) Dexamethasone eye drops containing γ-cyclodextrin-based nanogels. Int J Pharm 441:507–515
Nagarwal RC, Kant S, Singh PN, Maiti P, Pandit JK (2009) Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J Control Release 136:2–13
Natarajan JV, Darwitan A, Barathi VA, Ang M, Htoon HM, Boey F, Tam KC, Wong TT, Venkatraman SS (2014) Sustained drug release in nanomedicine: a long-acting nanocarrier-based formulation for glaucoma. ACS Nano 8:419–429
Nielsen CU, Brodin B, Jørgensen FS, Frokjaer S, Steffansen B (2002) Human peptide transporters: therapeutic applications. Expert Opin Ther Pat 12:1329–1350
Pan Q, Xu Q, Boylan NJ, Lamb NW, Emmert DG, Yang JC, Tang L, Heflin T, Alwadani S, Eberhart CG, Stark WJ, Hanes J (2015) Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J Control Release 201:32–40
Parveen S, Misra R, Sahoo SK (2012) Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 8:147–166
Patel VR, Agrawal YK (2011) Nanosuspension: An approach to enhance solubility of drugs. J Adv Pharm Technol Res 2:81
Patel P, Shastri D, Shelat P, Shukla A (2010) Ophthalmic drug delivery system: challenges and approaches. Syst Rev Pharm 1:113
Patel A, Cholkar K, Agrahari V, Mitra AK (2013) Ocular drug delivery systems: an overview. World J Pharmacol 2:47–64
Patravale VB, Date AA, Kulkarni RM (2014) Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol 56:827–840
Peynshaert K, Devoldere J, De Smedt SC, Remaut K (2018) In vitro and ex vivo models to study drug delivery barriers in the posterior segment of the eye. Adv Drug Deliv Rev 126:44–57
Pu X, Sun J, Li M, He Z (2009) Formulation of nanosuspensions as a new approach for the delivery of poorly soluble drugs. Curr Nanosci 5:417–427
Robinson JC (1993) Ocular anatomy and physiology relevant to ocular drug delivery. Drugs Pharm Sci 58:29–57
Rodriguez-Aller M, Guillarme D, El Sanharawi M, Behar-Cohen F, Veuthey JL, Gurny R (2013) In vivo distribution and ex vivo permeation of cyclosporine A prodrug aqueous formulations for ocular application. J Control Release 170:153–159
Saari KM, Nelimarkka L, Ahola V, Loftsson T, Stefansson E (2006) Comparison of topical 0.7% dexamethasone-cyclodextrin with 0.1% dexamethasone sodium phosphate for postcataract inflammation. Graefes Arch Clin Exp Ophthalmol 244:620–626
Sahoo SK, Dilnawaz F, Krishnakumar S (2008) Nanotechnology in ocular drug delivery. Drug Discov Today 13:144–151
Sai Y, Tsuji A (2004) Transporter-mediated drug delivery: recent progress and experimental approaches. Drug Discov Today 9:712–720
Sangwan VS, Pearson PA, Paul H, Comstock TL (2015) Use of the fluocinolone acetonide intravitreal implant for the treatment of noninfectious posterior uveitis: 3-year results of a randomized clinical trial in a predominantly Asian population. Ophthalmol Therapy 4:1–19
Scoper SV, Kabat AG, Owen GR, Stroman DW, Kabra BP, Faulkner R, Kulshreshtha AK, Rusk C, Bell B, Jamison T, Bernal-Perez LF, Brooks AC, Nguyen VA (2008) Ocular distribution, bactericidal activity and settling characteristics of TobraDex ST ophthalmic suspension compared with TobraDex ophthalmic suspension. Adv Ther 25:77–88
Senanayake P, Calabro A, Hu JG, Bonilha VL, Darr A, Bok D, Hollyfield JG (2006) Glucose utilization by the retinal pigment epithelium: evidence for rapid uptake and storage in glycogen, followed by glycogen utilization. Exp Eye Res 83:235–246
Sharom FJ (2011) The P-glycoprotein multidrug transporter. Essays Biochem 50:161–178
Shen Y, Tu J (2007) Preparation and ocular pharmacokinetics of ganciclovir liposomes. AAPS J 9:E371–E377
Shen J, Gan L, Zhu C, Zhang X, Dong Y, Jiang M, Zhu J, Gan Y (2011) Novel NSAIDs ophthalmic formulation: flurbiprofen axetil emulsion with low irritancy and improved anti-inflammation effect. Int J Pharm 412:115–122
Shende PK, Godbole R (2016) Current and novel techniques in the ophthalmic drug delivery systems. Int J Pharm Sci Res 7:3557–3566
Sigurdsson HH, Stefansson E, Gudmundsdottir E, Eysteinsson T, Thorsteinsdottir M, Loftsson T (2005) Cyclodextrin formulation of dorzolamide and its distribution in the eye after topical administration. J Control Release 102:255–262
Sigurdsson HH, Konraethsdottir F, Loftsson T, Stefansson E (2007) Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol Scand 85:598–602
Singh A, Li P, Beachley V, McDonnell P, Elisseeff JH (2015) A hyaluronic acid-binding contact lens with enhanced water retention. Cont Lens Anterior Eye 38:79–84
Soliman OAE, Mohamed EAM, El-Dahan MS, Khatera NAA (2017) Potential use of cyclodextrin complexes for enhanced stability, anti-inflammatory efficacy and ocular bioavailability of loteprednol etabonate. AAPS Pharm Sci Tech 18:1228–1241
Sutradhar KB, Khatun S, Luna IP (2013) Increasing possibilities of nanosuspension. J Nanotechnol 2013:346581
Taha EI, El-Anazi MH, El-Bagory IM, Bayomi MA (2014) Design of liposomal colloidal systems for ocular delivery of ciprofloxacin. Saudi Pharm J 22:231–239
Tajika T, Isowaki A, Sakaki H (2011) Ocular distribution of difluprednate ophthalmic emulsion 0.05% in rabbits. J Ocul Pharmacol Ther 27:43–49
Tiwari G, Tiwari R, Rai AK (2010) Cyclodextrins in delivery systems: applications. J Pharm Bioallied Sci 2:72–79
Tyagi P, Barros M, Stansbury JW, Kompella UB (2013) Light-activated, in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Mol Pharm 10:2858–2867
Urtti A (2006) Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev 58:1131–1135
Vadlapatla RK, Vadlapudi AD, Pal D, Mitra AK (2014) Role of membrane transporters and metabolizing enzymes in ocular drug delivery. Curr Drug Metab 15:680–693
Vaishya RD, Khurana V, Patel S, Mitra AK (2014) Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol 6:422–437
Valls R, Vega E, Garcia ML, Egea MA, Valls JO (2008) Transcorneal permeation in a corneal device of non-steroidal anti-inflammatory drugs in drug delivery systems. Open Med Chem J 2:66–71
Wadhwa S, Paliwal R, Paliwal SR, Vyas SP (2009) Nanocarriers in ocular drug delivery: an update review. Curr Pharm Des 15:2724–2750
Willoughby CE, Ponzin D, Ferrari S, Lobo A, Landau K, Omidi Y (2010) Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function—a review. Clin Exp Ophthalmol 38:2–11
Winkler BS (1981) Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol 77:667–692
Xie B, Jin L, Luo Z, Yu J, Shi S, Zhang Z, Shen M, Chen H, Li X, Song Z (2015) An injectable thermosensitive polymeric hydrogel for sustained release of Avastin(R) to treat posterior segment disease. Int J Pharm 490:375–383
Xuan M, Wang S, Liu X, He Y, Li Y, Zhang Y (2016) Proteins of the corneal stroma: importance in visual function. Cell Tissue Res 364:9–16
Yadollahi R, Vasilev K, Simovic S (2015) Nanosuspension technologies for delivery of poorly soluble drugs. J Nanomater 2015:1
Yasin MN, Svirskis D, Seyfoddin A, Rupenthal ID (2014) Implants for drug delivery to the posterior segment of the eye: a focus on stimuli-responsive and tunable release systems. J Control Release 196:208–221
Yellepeddi VK, Palakurthi S (2016) Recent advances in topical ocular drug delivery. J Ocul Pharmacol Ther 32:67–82
Yin J, Xiang C, Lu G (2016) Cationic lipid emulsions as potential bioadhesive carriers for ophthalmic delivery of palmatine. J Micoencapsul 33:718–724
Zhang R, He R, Qian J, Guo J, Xue K, Yuan YF (2010) Treatment of experimental autoimmune uveoretinitis with intravitreal injection of tacrolimus (FK506) encapsulated in liposomes. Invest Ophthalmol Vis Sci 51:3575–3582
Zou L, Nair A, Weng H, Tsai YT, Hu Z, Tang L (2011) Intraocular pressure changes: an important determinant of the biocompatibility of intravitreous implants. PLoS One 6:e28720
Acknowledgements
This work was financially supported by Grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2017R1C1B5015491 to Kyoung Ah Min and NRF-2015R1C1A1A02036781 to Meong Cheol Shin).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Maharjan, P., Cho, K.H., Maharjan, A. et al. Pharmaceutical challenges and perspectives in developing ophthalmic drug formulations. J. Pharm. Investig. 49, 215–228 (2019). https://doi.org/10.1007/s40005-018-0404-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-018-0404-6