Abstract
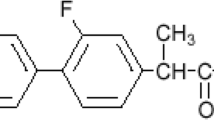
A novel dosage form integrating solid dispersion (SD) in orally disintegrating tablets (ODTs) was developed and optimized by the face-centered central composite design to improve poorly soluble property and slow onset action time of felodipine (Fel). SD of Fel and hydroxypropyl methylcellulose E6 was prepared by solvent evaporation method. Differential scanning calorimetry and fourier transforms infrared spectroscopy indicated that Fel transformed from crystalline to amorphous state by the formation of hydrogen bond between –N–H in Fel and O–R in HPMC. The accelerated stability test in 45 °C, 75 % RH demonstrated that the optimized SD was stable in terms of the dissolution rate of Fel and thermodynamic property. The ODTs containing SD (Fel:HPMC E6 = 1:3) were prepared by direct compression technique. The face-centered central composite design with the ODT-SD was employed to investigate the effect of mannitol (X1), crospovidone XL (X2) on the ODT-SD disintegration time (Y1), % Fel released after 5 min (Y2) and the ODT-SD friability (Y3). ANOVA test showed that X2 and X2 * X2 had a significant effect on the ODT-SD disintegration time (p < 0.05). Meanwhile, the dissolution rate of Fel after 5 min did not remarkably depend on any independent variables (p > 0.05). The ODT-SD friability was substantially proportional to the amount of mannitol (X1) (p < 0.05). The optimized ODT-SD disintegration time, % Fel released after 5 min, and friability were 27.67 s, 88.35 and 0.48 %, respectively.





Similar content being viewed by others
References
Abbou Oucherif K, Raina S, Taylor LS, Litster JD (2013) Quantitative analysis of the inhibitory effect of HPMC on felodipine crystallization kinetics using population balance modeling. CrystEngComm 15:2197–2205
Al-Khattawi A, Mohammed AR (2013) Compressed orally disintegrating tablets: excipients evolution and formulation strategies. Expert Opin Drug Deliv 10:651–663
Barnes PC, Bowles RML, Rajaratnam DV, Comerford MB, Ginks WR, Ginks SE, Goves JR, Grabau WJ, Malcolm JP, Hadley RJ, Hamilton DV, Hopkins RJ, Lawrie DM, Stoker JB, Morley CA, Taylor NC, Thompson FD, Kingswood JC, Elton RA, Forret EA (1988) Felodipine, a new calcium antagonist, as monotherapy in mild or moderate hypertension. Drugs 35:139–148
Basalious EB, El-Sebaie W, El-Gazayerly O (2013) Rapidly absorbed orodispersible tablet containing molecularly dispersed felodipine for management of hypertensive crisis: development, optimization and in vitro/in vivo studies. Pharm Dev Technol 18:407–416
Bolhuis GK, Rexwinkel EG, Zuurman K (2009) Polyols as filler-binders for disintegrating tablets prepared by direct compaction Polyols as filler-binders. Drug Dev Ind Pharm 35:671–677
Chiou WL, Riegelman S (1971) Pharmaceutical applications of solid dispersion systems. J Pharm Sci 60:1281–1302
Craig DQM (2002) The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm 231:131–144
Douroumis D (2011) Orally disintegrating dosage forms and taste-masking technologies; 2010. Expert Opin Drug Deliv 8:665–675
Hu X, Li Y, Zhang E, Wang X, Xing M, Wang Q, Lei J, Huang H (2013) Preparation and evaluation of orally disintegrating tablets containing taste-masked microcapsules of berberine hydrochloride. AAPS PharmSciTech 14:29–37
Katsuno E, Tahara K, Takeuchi Y, Takeuchi H (2013) Orally disintegrating tablets prepared by a co-processed mixture of micronized crospovidone and mannitol using a ball mill to improve compactibility and tablet stability. Powder Technol 241:60–66
Pandya VM, Patel DJ, Patel JK, Patel RP (2009) Formulation, characterization, and optimization of fast-dissolve tablets containing celecoxib solid dispersion. Dissolution Technol 16:22–27
Pfister WR, Ghosh TK (2005) Orally disintegrating tablets: products, technologies, and development issues. Pharm Technol 29:136–150
Risler T, Bohm R, Wetzchewald D, Nast HP, Koch HH, Stein G, Erley CM (1998) A comparison of the antihypertensive efficacy and safety of felodipine IV and nifedipine IV in patients with hypertensive crisis or emergency not responding to oral nifedipine. Eur J Clin Pharmacol 54:295–298
Rotthäuser B, Kraus G, Schmidt PC (1998) Optimization of an effervescent tablet formulation using a central composite design optimization of an effervescent tablet formulation containing spray dried l-leucine and polyethylene glycol 6000 as lubricants using a central composite design. Eur J Pharm Biopharm 46:85–94
Rumondor ACF, Stanford LA, Taylor LS (2009) Effects of polymer type and storage relative humidity on the kinetics of felodipine crystallization from amorphous solid dispersions. Pharm Res 26:2599–2606
Sano S, Iwao Y, Noguchi S, Kimura S, Itai S (2013) Design and evaluation of microwave-treated orally disintegrating tablets containing polymeric disintegrant and mannitol. Int J Pharm 448:132–141
Song Y, Wang L, Yang P, Wenslow RM, Tan B, Zhang H, Deng Z (2013) Physicochemical characterization of felodipine-kollidon VA64 amorphous solid dispersions prepared by hot-melt extrusion. J Pharm Sci 102:1915–1923
Teberekidis VI, Sigalas MP (2007) Theoretical study of hydrogen bond interactions of felodipine with polyvinylpyrrolidone and polyethyleneglycol. J Mol Struct (Thoechem) 803:29–38
Acknowledgments
This article does not contain any studies with human and animal subjects performed by any of the authors. All authors (N.-T. Tung, M.-V. Hung, X.-M. Vo, T.-H. Nguyen, T.-M.-H. Pham) declare that they have no conflict of interest. The authors are thankful to Department of Pharmaceutics, Hanoi University of Pharmacy for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tung, NT., Hung, MV., Vo, XM. et al. Formulation optimization of orally disintegrating tablets containing solid dispersion of felodipine and hydroxypropyl methylcellulose using face-centered central composite design. Journal of Pharmaceutical Investigation 44, 111–118 (2014). https://doi.org/10.1007/s40005-013-0106-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-013-0106-z