Abstract
Purpose
The dominant route of transmission of SARS-CoV-2 is airborne, through respiratory transmission by aerosols or droplets which can be measured by viral load in exhaled air. Several natural substances have shown antiviral activity. The aim of this pilot study was to investigate the effect of a chewing gum containing natural antiseptic ingredients (cinnamon-, peppermint- and lemon-oil, quercetin, spermidine, ginger and ginseng) on viral load in exhalative air in patients infected with SARS-CoV-2.
Methods
Nine patients infected with SARS-CoV-2 were enrolled and exhaled forcefully into a special mouthpiece at different time points before and after chewing the antiseptic gum. The mouthpiece contained a filter paper serving for extraction of coronaviruses following real-time PCR to quantify the viral load.
Results and conclusion
Cycle threshold (Ct) values of all patients increased after chewing the gum. The mean difference between the Ct values at baseline (before chewing the antiseptic gum) and time point 30 min (15 min after chewing) was 3.8 ± 2.6; (93% viral load reduction; p = 0.002). Time point 15 min (2.7 ± 1.7 (83% viral load reduction; p = 0.003)), 60 min (3.0 ± 3.4 (88% viral load reduction; p = 0.028)), 90 min (3.7 ± 1.8 (92% viral load reduction; p = 0.004)) and 120 min (3.0 ± 3.7 (91% viral load reduction; p = 0.05)) showed similar results. The antiseptic chewing gum demonstrated a significant potential to reduce SARS-CoV-2 viral load in exhalative air and, in this way, reduce further spread and infection risk. Larger placebo-controlled clinical trials are required to confirm these findings further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has turned in the current high impact pandemic. The dominant route of transmission of SARS-CoV-2 is airborne through respiratory transmission by aerosols or droplets [1].
The oral cavity is an important site for a SARS-CoV-2 infection, and saliva a potential route of SARS-CoV-2 transmission [2]. Oral viral load has been associated with severity of COVID-19 [3]. The current hygiene measures aim at reducing the spread of viral load from leaving the oral cavity. Antiseptic mouth rinses have shown promise to reduce viral load in the oral cavity and act as a preventive measure [4, 5]. However, they are rather complicated to use in different setups and populations. Depending on the situation, chewing gums or lozenges containing antiseptic volatile substances are easier to use than mouth rinses. Furthermore, they can be kept in the oral cavity for plenty of time and release their ingredients slowly and continuously. There are several antiseptic natural substances that have been shown to exhibit antiviral effects and seemed promising as potential ingredients for developing an antiviral chewing gum or lozenge [6].
The aim of this pilot trial was to investigate the effect of an antiviral chewing gum on viral load in exhalative air in patients infected with SARS-CoV-2.
Methods
The viral load in exhaled air was examined in patients infected with SARS-CoV-2 via PCR analysis before and after chewing an antiseptic chewing gum at different time points.
Cycle Threshold (Ct) values were generated by real-time PCR performed on the Cobas 6800 (Roche) detecting the ORF1 a/b and the E-gene of SARS-CoV-2 using the CE-certified test by Roche.
Before that, swabs had been sent to the Konsiliarlabor für Coronaviren (laboratory for corona virus) at the Institute of Virology, Charité-Universitätsmedizin Berlin, Germany for analyzing the Variants of Concern (VoC).
Subjects
Inclusion criteria: Volunteer adult (age over 18 years) patients suffering from COVID-19 with a positive PCR test confirming infection and being able to easily sit in an isolation room for two hours.
Study design
This prove of concept study was conducted at the IKF Clinical Research Center Respiratory Diseases, Frankfurt, Germany. PCR testing was done by Infektiologikum Frankfurt, Zentrum für fachübergreifende Infektionsmedizin (center for multidisciplinary infectious medicine). Approval of local ethics committee was granted; written consent was obtained, and the study was conducted in accordance with the declarations of Helsinki.
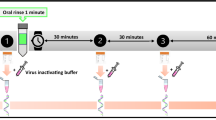
At each sample collection time point patients exhaled forcefully after a maximal inspiration through a mouthpiece containing a special filter paper. A predefined area of the filter paper was used for PCR analysis. The Ct value was used as marker for SARS-CoV-2 viral load. Ct is a theoretical parameter in PCR analysis. The Ct value shows the point in time at which a reliable measurement signal is present during the cyclic amplification: the lower the Ct value, the higher the amount of the target gene (corresponding nucleic acid) and thus the amount of virus particles. Conversely, a high Ct value indicates a low amount of nucleic acid and possibly a low virus titer.
Sample collection for the antiseptic chewing gum intervention occurred directly before (t = 0 min) and after a 15 min period (t = 15 min) of chewing, as well as 30 min (t = 30 min), 45 min (t = 45 min), 60 min (t = 60 min), 90 min (t = 90 min) and 120 min (t = 120 min) after the first sample collection.
Antiseptic gum active ingredients
In our study, a commercially available chewing gum (Clevergum GmbH, Munich, Germany) containing essential oils (cinnamon, lemon, peppermint) and extracts (ginger, ginseng, quercetin and spermidine) was used.
Statistical analysis
Main outcome parameter: Mean difference between the Ct values of time point 45 min (1/2 h after chewing) and baseline.
Further outcome parameters: Mean differences between each of the Ct values at the other time points after chewing (15 min, 30 min, 60 min, 75 min, 120 min) and baseline.
Differences between sample collection time points were evaluated using multiple paired samples t tests.
The global significance level was set to 0.05 and all statistical tests were two-sided. If not mentioned otherwise, mean values ± standard deviation is given.
Results
Nine patients infected with SARS-CoV-2 between 18 and 64 years took part in this pilot trial. All patients were symptomatic for at least one day and a maximum of six days. Four patients had wild type, three alpha and two delta SARS-CoV-2 variants.
The Ct values before chewing the gum (baseline, time point 0) were 29.9 ± 2.5, and increased in all patients after chewing the gum.
The mean difference between the Ct values at baseline (time point 0; before chewing the antiseptic gum) and time point 30 min (15 min after chewing) was 3.8 ± 2.6; (93% viral load reduction; p = 0.002). Figure 1 shows the viral load reduction as compared to baseline in terms of differences of Ct values to baseline as error bars (mean ± SD) for all time points and over all patients. All means lie over the zero line, which indicates viral load reduction.
When compared with baseline mean difference between the Ct values at time point 15 min (directly after chewing) was 2.7 ± 1.7 (83% viral load reduction; p = 0.003), at time point 45 min (1/2 h after chewing) 3.1 ± 0.7 (88% viral load reduction; p < 0.001), at time point 60 min 3.0 ± 3.4 (88% viral load reduction; p = 0.028), at time point 90 min 3.7 ± 1.8 (92% viral load reduction; p = 0.004) and at time point 120 min 3.0 ± 3.7 (91% viral load reduction; p = 0.05) (Fig. 1). Individual patient trends are shown in Fig. 2.
Discussion
Viral load in exhaled air is supposedly a representative parameter for infection risk: Assessing the infectiousness of COVID-19 patients merely through pharyngeal swabs seems to not be accurate whereas exhaled breath was concluded to represent a more suitable matrix for evaluating the degree of infectiousness [7].
The active ingredients of the antiseptic gum have all shown to exhibit antiseptic effects. Cinnamon oil [8,9,10,11,12], extract [20,21,22] and ginger extract [9, 22] as well as spermidine [23] have shown strong antiseptic effects and peppermint oil [13, 14], citrus oil [12, 15,16,17], the flavonoid quercetin [18, 19] as well as saponin containing panax ginseng have shown antiviral effects in vitro. Lemon essential oils furthermore downregulate angiotensin-converting enzyme2 (ACE2), a SARS-CoV-2 spike receptor-binding domain in epithelial cells [24].
In this pilot study, patients that had chewed antiseptic chewing gums showed a significant reduction of viral load in exhaled air, indicated by increased Ct values as compared to baseline, for up to 120 min. Given a reduction of 50% of viral load per 1.0 increase of Ct value, viral load dropped between 84 and 93% on average within a 2 h period after intake of the chewing gum. These results point to a significant antiviral effect of the antiseptic chewing gum confirming the antiviral in vitro effect of its antiseptic ingredients.
The antiseptic gum used in this study is the first of such products on the market, so there is no comparable food product.
Mouth rinses developed for antiseptic mouth washing containing for example povidone iodide or chlorhexidine have shown promising effects on SARV-CoV-2 in vitro [25], however in vivo evidence is still limited and studies in COVID-19 patients examining exhaled air are lacking so far [4].
There are some limitations of this pilot study. We included a relatively small number of patients which showed expected individual trends of viral load reduction. This trial was designed as a “proof-of-principle”-study to investigate whether chewing an antiseptic gum can significantly impact reduction of viral load in exhaled air at all. It is further to mention that only wild type, alpha and delta variants were included. This is due to the fact, that the pilot trial was performed when no omicron variants were yet present in Germany. There may possibly be differences in the anatomic replication region for the different SARS-COV-2 variants. However, we expect an equal distribution of antiseptic gum ingredients in all relevant potential replication sites due to the continuous chewing process.
Another interesting question to address is whether chewing a gum by itself may—independent from the gum’s ingredients—increase saliva production and dilute or decrease viral load. We report a nearly tenfold reduction of viral load in exhaled air and do not consider increased saliva flow in the oral cavity solely or largely responsible for such a result.
In addition, an increased saliva flow in the oral cavity by chewing any type of gum will most probably not influence viral load measured in exhaled air to such an extend measured as in our study.
Despite that, we believe that it is necessary to rule out dilution or other effects by including a control group in future studies. In order to take into account the limitations of this pilot trial we have just started a larger placebo controlled trial including patients suffering from infection with omicron variants (trial number 2022-2870-evBO).
Conclusion
The antiseptic chewing gum showed promise to reduce SARS-CoV-2 viral load in exhalative air and, by doing so, reduce infection risk. Larger placebo-controlled clinical trials will need to confirm these findings further.
Availability of data and materials
Study carried out at IKF Frankfurt. All data and material can be accessible on the website.
References
Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2021;174:69–79. https://doi.org/10.7326/M20-5008.
Huang N, Perez P, Kato T, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27:892–903. https://doi.org/10.1038/s41591-021-01296-8.
Herrera D, Serrano J, Roldan S, Sanz M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin Oral Investig. 2020;24:2925–30. https://doi.org/10.1007/s00784-020-03413-2.
Mateos-Moreno MV, Mira A, Ausina-Marquez V, Ferrer MD. Oral antiseptics against coronavirus: in-vitro and clinical evidence. J Hosp Infect. 2021;113:30–43. https://doi.org/10.1016/j.jhin.2021.04.004.
Vergara-Buenaventura A, Castro-Ruiz C. Use of mouthwashes against COVID-19 in dentistry. Br J Oral Maxillofac Surg. 2020;58:924–7. https://doi.org/10.1016/j.bjoms.2020.08.016.
Asif M, Saleem M, Saadullah M, Yaseen HS, Al ZR. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology. 2020;28:1153–61. https://doi.org/10.1007/s10787-020-00744-0.
Malik M, Kunze AC, Bahmer T, Herget-Rosenthal S, Kunze T. SARS-CoV-2: viral loads of exhaled breath and oronasopharyngeal specimens in hospitalized patients with COVID-19. Int J Infect Dis. 2021;110:105–10. https://doi.org/10.1016/j.ijid.2021.07.012.
Bassyouni RH, Kamel Z, Abdelfattah MM, Mostafa E. Cinnamon oil: a possible alternative for contact lens disinfection. Cont Lens Anterior Eye. 2016;39:277–83. https://doi.org/10.1016/j.clae.2016.01.001.
Bauer Faria TR, Furletti-Goes VF, Franzini CM, et al. Anti-inflammatory and antimicrobial effects of Zingiber officinale mouthwash on patients with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2021;159:21–9. https://doi.org/10.1016/j.ajodo.2019.10.025.
Mamajiwala AS, Sethi KS, Raut CP, Karde PA, Khedkar SU. Comparative evaluation of chlorhexidine and cinnamon extract used in dental unit waterlines to reduce bacterial load in aerosols during ultrasonic scaling. Indian J Dent Res. 2018;29:749–54. https://doi.org/10.4103/ijdr.IJDR_571_17.
Utchariyakiat I, Surassmo S, Jaturanpinyo M, Khuntayaporn P, Chomnawang MT. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement Altern Med. 2016;16:158. https://doi.org/10.1186/s12906-016-1134-9.
Mani JS, Johnson JB, Steel JC, et al. Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res. 2020;284: 197989. https://doi.org/10.1016/j.virusres.2020.197989.
Thosar N, Basak S, Bahadure RN, Rajurkar M. Antimicrobial efficacy of five essential oils against oral pathogens: an in vitro study. Eur J Dent. 2013;7:S071–7. https://doi.org/10.4103/1305-7456.119078.
Schuhmacher A, Reichling J, Schnitzler P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine. 2003;10:504–10. https://doi.org/10.1078/094471103322331467.
Ebani VV, Najar B, Bertelloni F, Pistelli L, Mancianti F, Nardoni S. Chemical composition and in vitro antimicrobial efficacy of sixteen essential oils against Escherichia coli and Aspergillus fumigatus isolated from poultry. Vet Sci. 2018. https://doi.org/10.3390/vetsci5030062.
Laird K, Kurzbach E, Score J, Tejpal J, Chi Tangyie G, Phillips C. Reduction of Legionella spp. in water and in soil by a citrus plant extract vapor. Appl Environ Microbiol. 2014;80:6031–6. https://doi.org/10.1128/AEM.01275-14.
Meneguzzo F, Ciriminna R, Zabini F, Pagliaro M (2020) Hydrodynamic cavitation-based rapid expansion of hesperidin-rich products from waste citrus peel as a potential tool against COVID-19. Preprints
Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front Immunol. 2020;11:1451. https://doi.org/10.3389/fimmu.2020.01451.
Kim CH, Kim JE, Song YJ. Antiviral activities of quercetin and isoquercitrin against human herpesviruses. Molecules. 2020. https://doi.org/10.3390/molecules25102379.
Lim DS, Bae KG, Jung IS, Kim CH, Yun YS, Song JY. Anti-septicaemic effect of polysaccharide from Panax ginseng by macrophage activation. J Infect. 2002;45:32–8. https://doi.org/10.1053/jinf.2002.1007.
Wang Y, Jung YJ, Kim KH, et al. Antiviral activity of fermented ginseng extracts against a broad range of influenza viruses. Viruses. 2018. https://doi.org/10.3390/v10090471.
Metwaly AM, Lianlian Z, Luqi H, Deqiang D. Black ginseng and its Saponins: preparation, phytochemistry and pharmacological effects. Molecules (Basel, Switzerland). 2019;24:1856. https://doi.org/10.3390/molecules24101856 (In eng).
Gassen NC, Papies J, Bajaj T, et al. Analysis of SARS-CoV-2-controlled autophagy reveals spermidine, MK-2206, and niclosamide as putative antiviral therapeutics. bioRxiv. 2020. https://doi.org/10.1101/2020.04.15.997254.
Senthil Kumar KJ, Gokila Vani M, Wang CS, et al. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain epithelial cells. Plants (Basel). 2020. https://doi.org/10.3390/plants9060770.
Seneviratne CJ, Balan P, Ko KKK, et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection. 2021;49(2):305–11. https://doi.org/10.1007/s15010-020-01563-9.
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript. KO, SM, and B-JB developed the study design and performance, AD did the laboratory analysis. KM was responsible for the statistics.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
Study methodology approved by local ethics committee (Landesärztekammer Hessen).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pfab, F., Buelow-Johansen, B., Alber, D. et al. Reduction of SARS-CoV-2 viral load in exhaled air by antiseptic chewing gum: a pilot trial. Infection 51, 881–885 (2023). https://doi.org/10.1007/s15010-022-01944-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-022-01944-2