Abstract
Background
Cryptococcal infection has been increasingly reported in patients with COVID-19 infection, but the epidemiological factors, presentation, diagnostic certainty, and outcome have not been well-described.
Methods
We reviewed the published cases of COVID-19-associated Cryptococcus infections (CACI) to shed the light on the burden of this infection.
Results
We identified 13 patients with confirmed cryptococcal infection. Cryptococcus infection was primarily seen in patients with severe COVID-19 disease who received corticosteroids therapy and admitted to the intensive care unit. Pulmonary CACI was the most common reported infection followed by cryptococcal meningitis.
Conclusion
In light of the high mortality rate, clinicians should maintain a high clinical suspicion of CACI in critically ill patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2019 Novel Coronavirus Disease (COVID-19) emerged in Wuhan, China in 2019, and as of September 2021, there have been 238 million people infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with 4.8 million confirmed deaths worldwide. This pandemic continues swirling around the globe and has significantly impacted on the healthcare systems. Several studies reported the relatively common COVID-19-associated co-infections whether secondary to bacteria, viruses, or fungal pathogens [1]. In addition, critically ill patients, who required mechanical ventilation or had prolonged hospital stay, were more likely to develop fungal co-infections [2]. Severe COVID-19 disease is associated with increased pro-inflammatory cytokines, interleukin (IL)-1, IL-6, and tumor necrosis factor alpha, reduced CD4-interferon-gamma expression, CD4 and CD8 T cells, which all increase the susceptibility to fungal infections [2]. Fungal co-infection secondary to Aspergillus and Candida species was reported in severely ill COVID-19 patients in up to 5.8% of cases [3]. Similarly, a prospective study has demonstrated the high incidence of COVID-19-associated pulmonary aspergillosis (CAPA) among critically ill COVID-19 patients [4]. In addition, during the severe surge of COVID-19 infection in India, COVID-19-associated mucormycosis (CAM) cases were reported and this opportunistic infection may be due to the alignment of multiple traditional risk factors such as diabetes mellitus (DM) on top of lung tissue damage induced by SARS-CoV-2 infection and possibly environmental exposures [5, 6].
More recently, cryptococcal infections have emerged as a matter of concern following COVID-19 infection and interestingly, Cryptococcus spores can remain dormant for many years in human hosts and may lead to invasive cryptococcosis and dissemination in the settings of corticosteroids use, immunosuppressive therapy, DM, and malignancies [7, 8]. At present, few cases of Cryptococcus infection were reported in COVID-19 patients. Herein, we reviewed all reported cases of CACI and discussed their risk factors, epidemiology, and clinical outcomes.
Methods
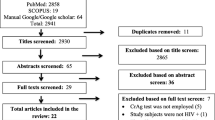
We searched PubMed until January 21st, 2022 using the search terms “COVID-19” or “SARS-CoV-2” and “Cryptococcus” or “Cryptococcosis”. We included only cases which contained adequate data and medical information about risk factors, clinical presentation, clinical course, diagnosis, treatment, and outcomes [9,10,11,12,13,14,15,16,17,18,19,20,21]. We identified 15 cases of COVID-19-associated cryptococcal infections, but two patients were excluded, as they were diagnosed with cryptococcosis prior to COVID-19 infection [22, 23]. Proven cryptococcal infection was defined as per EORTC-MSG criteria [24]. Confirmed cases have either positive culture of a sample obtained from a normally sterile site showing a clinical or radiological findings consistent with an infectious disease process, positive blood culture, or positive cryptococcal antigen in cerebrospinal fluid (CSF) or blood.
Results
We identified 13 patients with confirmed cryptococcal infection. Patients’ characteristics and infections details are summarized in Tables 1 and 2. Ten patients (76.9%) were males with a median age of 73 years [interquartile (IQR), 24–78]. Out of 13 patients, 5 (38.4%) were diabetic and 9 (69.2%) had hypertension. Twelve patients (92.3%) had severe COVID-19 disease and one had mild infection. Eleven patients (84.6%) presented with shortness of breath, 6 patients (46.1%) had fever, 13 patients (100%) required ICU admission during their hospital stays and 12 patients (92.3%) required mechanical ventilation. Six patients (46.1%) received remdesivir and 3 patients (23%) received tocilizumab. Twelve patients (92.3%) received corticosteroids equivalent to prednisone 10 mg/day for more than 7 days. In 11 patients, the median time duration between COVID-19 diagnosis and Cryptococcus infection was 13 days (IQR, 5–49) and 2 patients (15.3%) were diagnosed postmortem. Seven patients (53.8%) had new fever at the time of cryptococcal infection, whereas 4 patients (30.7%) had respiratory insufficiency, and 2 patients (15.3%) developed septic shock. Four patients (30.7%) had also neurologic symptoms at the time of Cryptococcus infection. Of interest, 8 patients (61.5%) had positive blood culture for Cryptococcus, where six (46.1%) patients had positive CSF cultures for Cryptococcus and 3 patients (23%) had positive bronchoalveolar lavage fluid (BAL) culture. Pulmonary cryptococcal infection was seen in 7 patients (53.8%), whereas cryptococcal meningitis was seen in 4 patients (30.7%). Disseminated cryptococcosis with lung and meningeal involvement was seen in 2 patients (15.3%). Among the 6 patients who had cryptococcal meningitis, CSF culture was positive for Cryptococcus in all 6 patients (100%), whereas cryptococcal antigen test was done only in 3 patients. Out of 13 patients, cryptococcal serology was done in only 2 patients (serum antigen), which was positive in one patient. Eleven patients (84.6%) received antifungal treatment; 9 were treated with liposomal amphotericin B (L-AmB) and flucytosine; one patient was initially treated with L-AmB but later changed to isavuconazole due to acute kidney injury. One patient treated only with L-AmB. In-hospital mortality was observed in 7 patients (53.8%) and one patient remains comatose at the time of publication. Postmortem diagnosis was made in 2 patients (15.3%). The mean time duration between cryptococcal diagnosis and death was 23 days. Five patients (38.4%) were successfully discharged. For instance, mortality among patients who received antifungal treatment was seen in 5/11 patients (45.4%).
Discussion
Although our review is subject to publication biases, several observations emerged. First, we have only 13 cases of CACI who were initially diagnosed with COVID-19 and subsequently developed cryptococcal infection. Our analysis showed that most patients were elderly with median age of 73 years and presented with severe COVID-19 infection required mechanical ventilation and ICU admissions. Up to 92.3% of patients received corticosteroids and three patients received tocilizumab. Importantly, cryptococcal infection occurred relatively early after COVID-19 diagnosis (13 days, median). Most of the patients had pulmonary cryptococcal infection and their course were complicated with prolonged hospital stays. In contrast to COVID-19 patients, the common underlying conditions that have been relayed to increased risk of pulmonary cryptococcosis include HIV infection, malignancies, stem cell and solid organ transplantation, liver cirrhosis, renal failure with proteinuria and treatment with glucocorticoids or tumor necrosis factor-alpha antagonists [25].
The increase risk of fungal infections in COVID-19 patients are often related to high doses and prolonged therapy of corticosteroids [26]. The Infectious Diseases Society of America (IDSA) COVID-19 guidelines currently recommend use of dexamethasone 6 mg IV or PO for 10 days or until discharge, whichever is sooner. The benefits from using dexamethasone in COVID-19 include 34% reduction of mortality at 28 days among hospitalized, critically ill patients compared to those not treated with glucocorticoids, as well as patients receiving dexamethasone were more likely to be discharged from the hospital at 28 days [27]. It is vital, however, to balance risks versus benefits by avoiding unnecessary large doses or prolonged duration of corticosteroids therapy.
In addition, the Food and Drug Administration (FDA) authorized the use of tocilizumab in June 2021 to serve as another layer of fighting against COVID-19 infection. This emergency use authorization (EUA) contains a warning of an increased risk of serious infections, including active tuberculosis and invasive fungal, bacterial, and other opportunistic infections, primarily when tocilizumab is concomitantly used with other immunosuppressive drugs, including corticosteroids. However, under the EUA in the setting of COVID-19, tocilizumab is indicated for patients that are also receiving systemic corticosteroids and requiring supplemental oxygen, therefore increasing the patient’s risk of serious infection [28, 29]. Historically tocilizumab has mostly been used for rheumatoid arthritis, and there are select case reports detailing cryptococcal infection resulting from tocilizumab use in this setting. It has been reported that the incidence rate of cryptococcosis in patients receiving tocilizumab is around 0.04%. The significant risk factors for serious infection in patients who are receiving tocilizumab include age ≥ 65 years, respiratory disease, and corticosteroid use with prednisone equivalent ≥ 5 mg/day [30].
More recently, Baricitinib has also been approved for COVID-19 treatment [31]. It is a janus kinase (JAK) inhibitor, inhibiting cytokines such as IL-2, IL-6, IL-10, and interferon-gamma and halt the exaggerated immune response. However, another infectious concern has been raised, such as reactivation of latent viruses (herpes simplex virus) following the treatment with JAK inhibitors. Winthrop and colleagues mentioned reactivation of several cases of cytomegalovirus, with less frequent reports of reactivation of latent tuberculosis [32]. Although, Kalil et al. concluded that the addition of baricitinib to remdesivir was not associated with a significantly higher incidence of adverse events [33].
Extrapolating from influenza A virus infection, it has been purported based on experimental murine model that influenza viral infection itself may be a predisposing factor for cryptococcosis by causing immune dysregulation, lung tissue damage, and disruption of phagocytosis leading to further fungal proliferation and severe disease [34]. Few case reports have been published on severe influenza and cryptococcal infections [35]. In addition, SARS-CoV-2 can affect the JAK/STAT pathway and renders the infected cells resistant to type I interferon which can further contribute to increased risk of opportunistic infection, such as cryptococcosis [36]. All in all, taking into consideration the immunosuppressed status resulted from the SARS-CoV-2 infection and add on the effects of immunomodulating and immunosuppressed drugs that are given for severely ill COVID-19 patients, opportunistic infections (OIs) will continue on the rise and clinicians should be aware of these OIs by keeping a low threshold for an early diagnosis and prompt therapy to reduce the morbidity and mortality rate related to COVID-19 infection.
Conclusions
While the pathogenesis of cryptococcal infection following COVID-19 remains under investigation, SARS-CoV-2-driven etiology intertwined with glucocorticoid therapy, mechanical ventilation, critical illness appeared as risk factors. Clinicians should maintain a high index of suspicion and keep CACI in the differential diagnosis of severely ill patients with COVID-19 infection, especially in case of clinical deterioration and worsening respiratory status.
References
Lai C-C, Wang C-Y, Hsueh P-R. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect. 2020;53:505–12.
Pemán J, Ruiz-Gaitán A, García-Vidal C, et al. Fungal co-infection in COVID-19 patients: should we be concerned? Rev Iberoam micol. 2020;37:41–6.
Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81.
Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. 2021;73(11):e3606–14.
Narayanan S, Chua JV, Baddley JW. Coronavirus disease 2019–associated mucormycosis: risk factors and mechanisms of disease. Clin Infect Dis. 2021;22:ciab726.
John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi (Basel, Switzerland). 2021;7(4):298.
Setianingrum F, Rautemaa-Richardson R, Denning DW. Pulmonary cryptococcosis: a review of pathobiology and clinical aspects. Med Mycol. 2019;57:133–50.
Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179–206.
Thyagarajan RV, Mondy KE, Rose DT. Cryptococcus neoformans blood stream infection in severe COVID-19 pneumonia. IDCases. 2021;26:e01274.
Gamon E, Tammena D, Wattenberg M, Augenstein T. Rare superinfection in a COVID-19 patient-A chronology. Anaesthesist. 2021;24:1–12.
Ghanem H, Sivasubramanian G. Cryptococcus neoformans meningoencephalitis in an immunocompetent patient after COVID-19 infection. C Rep Infect Dis. 2021;2021:5597473.
Khatib MY, Ahmed AA, Shaat SB, Mohamed AS, Nashwan AJ. cryptococcemia in a patient with COVID-19: a case report. Clin C Rep. 2021;9:853–5.
Heller HM, Gonzalez RG, Edlow BL, Ard KL, Gogakos T. Case 40–2020: a 24-year-old man with headache and COVID-19. N Engl J Med. 2020;383:2572–80.
Passarelli VC, Perosa AH, de Souza Luna LK, et al. Detected SARS-CoV-2 in ascitic fluid followed by cryptococcemia: a case report. SN Compr Clin Med. 2020. https://doi.org/10.1007/s42399-020-00574-9.
Cafardi J, Haas D, Lamarre T, Feinberg J. Opportunistic fungal infection associated with COVID-19. Open Forum Infect Dis. 2021. https://doi.org/10.1093/ofid/ofab016.
Abohelwa MM, del Rio-Pertuz G, Parmar KN, et al. Pulmonary cryptococcosis in the 2019 novel coronavirus, when the coinfection affects the mortality. Am J Respir Crit Care Med. 2021;203:A2461.
Alegre-González D, Herrera S, Bernal J, Soriano A, Bodro M. Disseminated cryptococcus neoformans infection associated to COVID-19. Med Mycol C Rep. 2021;34:35–7.
Traver EC, Malavé SM. Pulmonary aspergillosis and cryptococcosis as a complication of COVID-19. Med Mycol C Rep. 2022;35:22–5.
Thota DR, Ray B, Hasan M, Sharma K. Cryptococcal meningoencephalitis during convalescence from severe COVID-19 pneumonia. Neurohospitalist. 2022;12:96–9.
Karnik K, Wu Y, Ruddy S, et al. Fatal case of disseminated cryptococcal infection and meningoencephalitis in the setting of prolonged glucocorticoid use in a COVID-19 positive patient. IDCases. 2022;27:e01380.
Gil Y, Gil YD, Markou T. The emergence of cryptococcemia in COVID-19 Infection: a case report. Cureus. 2021;13:e19761.
Chiappe Gonzalez AJ, Montenegro-Idrogo JJ, Vargas Vadillo AR, Slee Torres M, Vargas Matos I, Resurrección Delgado CP. Hospital-acquired SARS-CoV-2 pneumonia in a person living with HIV. Int J STD AIDS. 2020;31:1320–2.
Passerini M, Terzi R, Piscaglia M, Passerini S, Piconi S. Disseminated cryptococcosis in a patient with metastatic prostate cancer who died in the coronavirus disease 2019 (COVID-19) Outbreak. Cureus. 2020;2020:12.
Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis Publ Infect Dis Soc Am. 2020;71:1367–76.
Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50:291–322.
Ezeokoli OT, Gcilitshana O, Pohl CH. Risk factors for fungal co-infections in critically ill COVID-19 patients, with a focus on immunosuppressants. J Fungi (Basel, Switzerland). 2021. https://doi.org/10.3390/jof7070545.
Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa478.
Fact sheet for healthcare providers: emergency use authorization for Actemra ® (tocilizumab) highlights of emergency use authorization (EUA) these highlights of the EUA do not include all the information needed to use Actemra under the EUA. see the full fact sheet for healthcare providers for Actemra. Actemra ® (tocilizumab) injection, for intravenous use Original EUA Authorized Date: 06/2021. Available at: www.clinicaltrials.gov. Accessed 30 October 2021.
Thota DR, Ray B, Hasan M, Sharma K. Cryptococcal meningoencephalitis during convalescence from severe COVID-19 pneumonia. Neurohospitalist. 2021. https://doi.org/10.1177/19418744211009766.
Nishioka H, Takegawa H, Kamei H. Disseminated cryptococcosis in a patient taking tocilizumab for Castleman’s disease. J Infect Chemother: official J Japan Soc Chemother. 2018;24:138–41.
Adarsh Bhimraj A, Morgan RL, Hirsch Shumaker A, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Available at: www.idsociety.org/COVID19guidelines. Accessed 30 Oct 2021.
Winthrop KL, Harigai M, Genovese MC, et al. Infections in baricitinib clinical trials for patients with active rheumatoid arthritis. Ann Rheum Dis. 2020;79:1290–7.
Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795–807.
Oliveira LVN, Costa MC, Magalhães TFF, et al. Influenza a virus as a predisposing factor for cryptococcosis. Front Cell Infect Microbiol. 2017;7:419.
Huang J, Li H, Lan C, et al. Concomitant severe influenza and cryptococcal infections: a case report and literature review. Medicine. 2019;98:e15544.
Chen D-Y, Khan N, Close BJ, et al. SARS-CoV-2 disrupts proximal elements in the JAK-STAT pathway. J Virol. 2021;95:e0086221.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
All authors contribute to writing and editing of the manuscript. AEM structured the main ideas of the paper.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Rights and permissions
About this article
Cite this article
Regalla, D., VanNatta, M., Alam, M. et al. COVID-19-associated Cryptococcus infection (CACI): a review of literature and clinical pearls. Infection 50, 1007–1012 (2022). https://doi.org/10.1007/s15010-022-01805-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-022-01805-y