Summary
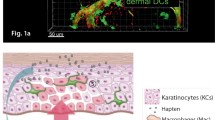
The skin is equipped with serial barriers that provide rapid and efficient protection against external intruders. Beneath the epidermal physical barriers of the stratum corneum and the tight junctions, the integrated immune systems in both the epidermis and the dermis act in a coordinated manner to protect the host. This „immunological“ barrier is composed of various cells, including skin-resident cells, such as keratinocytes, dendritic cells, tissue-resident macrophages, resident memory T cells, mast cells, and innate lymphoid cells. Additionally, infiltrating memory T cells, monocytes, neutrophils, basophils, and eosinophils are recruited in support of the host immunity.
In addition to discussing the role of each of these cellular populations, we describe the concept of skin associated lymphoid tissue (SALT), which reminds us that the skin is an important component of the lymphatic system. We further describe the newly discovered phenomenon of multiple cell gathering under skin inflammation, which can be referred to as inducible SALT (iSALT). iSALT contributes to our understanding of SALT by highlighting the importance of direct cell-cell interaction in skin immunity.
Cite this as Ono S, Kabashima K. Novel insights into the role of immune cells in skin and inducible skin-associated lymphoid tissue (iSALT). Allergo J Int 2015;24:170–9 DOI: 10.1007/s40629-015-0065-1
Similar content being viewed by others
Abbreviations
- AD:
-
Atopic dermatitis
- Ags:
-
Antigens
- AMPs:
-
Anti-microbial peptides
- APs:
-
Antigen presenting cells
- ASC:
-
Apoptosis-associated speck like protein containing a caspase recruitment domain
- ATP:
-
Adenosine triphosphate
- CCL5:
-
Chemokine C-C motif ligand 5
- CHS:
-
Contact hypersensitivity
- CXCL2:
-
Chemokine C-X-C motif ligand 2
- CX3CR1:
-
C-X3-C chemokine receptor 1
- CXCR2:
-
Chemokine C-X-C motif receptor 2
- DAMPs:
-
Damage-associated molecular patterns
- cDCs:
-
Dermal conventional DCs
- dDCs:
-
Dermal DCs
- DCs:
-
Dendritic cells
- dLNs:
-
Draining lymph nodes
- DN:
-
Double negative
- dSEARCH:
-
Dendrite surveillance extension and retraction cycling habitude
- DTA:
-
Diphtheria toxin subunit A
- DTR:
-
Diphtheria toxin receptor
- HMGB1:
-
High-mobility group box protein 1
- HSV-2:
-
Herpes simplex virus
- IFN:
-
Interferon
- IL:
-
Interleukin
- ILCs:
-
Innate lymphoid cells
- iSALT:
-
Inducible SALT
- KCs:
-
Keratinocytes
- LCs:
-
Langerhans cells
- LPS:
-
Lipopolysaccharide
- M1:
-
Classically activated macrophage
- M2:
-
Alternatively activated macrophage
- MALT:
-
Mucosa-associated lymphoid tissue
- Mgl2:
-
Macrophage galactose C-type lectin type 2
- MHC:
-
Major histocompatibility complex
- MLCs:
-
Memory lymphocyte clusters
- NLR:
-
Nucleotide-binding domain and leucine-rich repeat containing family
- PAMPs:
-
Pathogen-associated molecular patterns
- pDCs:
-
Plasmacytoid dendritic cells
- PRRs:
-
Pattern recognition receptors
- SALT:
-
Skin associated lymphoid tissue
- SC:
-
Stratum corneum
- Th:
-
T helper
- TJs:
-
Tight junctions
- TLRs:
-
Toll-like receptors
- TNF:
-
Tumor necrosis factor
- TSLP:
-
Thymic stromal lymphopoietin
- XCR1:
-
XC-chemokine receptor 1
References
Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci 2013;70:3–11
Streilein JW. Skin-associated lymphoid tissues (SALT): origins and functions. J Invest Dermatol 1983; 80 Suppl: 12s–16s
Natsuaki Y, Egawa G, Nakamizo S, Ono S, Hanakawa S, Okada T, et al. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat Immunol 2014;15:1064–1069
Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol 2009;9:679–691
Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol 2007;127:331–341
Begon E, Michel L, Flageul B, Beaudoin I, Jean-Louis F, Bachelez H, et al. Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur J Dermatol 2007;17:497–506
Selleri S, Arnaboldi F, Palazzo M, Gariboldi S, Zanobbio L, Opizzi E, et al. Toll-like receptor agonists regulate beta-defensin 2 release in hair follicle. Br J Dermatol 2007;156:1172–1177
Muller-Anstett MA, Muller P, Albrecht T, Nega M, Wagener J, Gao Q, et al. Staphylococcal peptidoglycan co-localizes with Nod2 and TLR2 and activates innate immune response via both receptors in primary murine keratinocytes. PLoS One 2010;5:e13153
Sugita K, Kabashima K, Atarashi K, Shimauchi T, Kobayashi M, Tokura Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clin ExpImmunol 2007;147:176–183
Miller LS, Modlin RL. Human keratinocyte Toll-like receptors promote distinct immune responses. J Invest Dermatol 2007;127:262–263
Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002;3:673–680
Hirsiger S, Simmen HP, Werner CM, Wanner GA, Rittirsch D. Danger signals activating the immune response after trauma. Mediators Inflamm 2012; 2012: 315941
Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol 2009;27:229–265
Fukata M, Vamadevan AS, Abreu MT. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol 2009;21:242–253
Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol 2007;17:1140–1145
Burrell HE, Wlodarski B, Foster BJ, Buckley KA, Sharpe GR, Quayle JM, et al. Human keratinocytes release ATP and utilize three mechanisms for nucleotide interconversion at the cell surface. J Biol Chem 2005;280:29667–29676
Nakajima S, Watanabe H, Tohyama M, Sugita K, Iijima M, Hashimoto K, et al. High-mobility group box 1 protein (HMGB1) as a novel diagnostic tool for toxic epidermal necrolysis and Stevens-Johnson syndrome. Arch Dermatol 2011;147:1110–1112
Mattii M, Ayala F, Balato N, Filotico R, Lembo S, Schiattarella M, et al. The balance between pro- and anti-inflammatory cytokines is crucial in human allergic contact dermatitis pathogenesis: the role of IL-1 family members. Exp Dermatol 2013;22:813–819
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 recep-tor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479–490
Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006;203:2271–2279
Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature 1997;387:861
Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol 1995;32:982–986
de Jongh GJ, Zeeuwen PL, Kucharekova M, Pfundt R, van der Valk PG, Blokx W, et al. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol 2005;125:1163–1173
Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002;347:1151–1160
Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007;449:564–569
Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol 2005; 17: 273–283
Hunger RE, Sieling PA, Ochoa MT, Sugaya M, Burdick AE, Rea TH, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest 2004;113:701–708
Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol 2006;126:787–796
Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med 2009;206:2937–2946
Nakajima S, Igyarto BZ, Honda T, Egawa G, Otsuka A, Hara-Chikuma M, et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol 2012;129:1048–1055 e6
Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol 2008;38:2369–2376
Igyarto BZ, Jenison MC, Dudda JC, Roers A, Muller W, Koni PA, et al. Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10. J Immunol 2009;183:5085–5093
Yoshiki R, Kabashima K, Sugita K, Atarashi K, Shimauchi T, Tokura Y. IL-10-producing Langerhans cells and regulatory T cells are responsible for depressed contact hypersensitivity in grafted skin. J Invest Dermatol 2009;129:705–713
Gomez de Aguero M, Vocanson M, Hacini-Rachinel F, Taillardet M, Sparwasser T, Kissenpfennig A, et al. Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8(+) T cells and activating Foxp3(+) regulatory T cells. J Clin Invest 2012;122:1700–1711
Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol 2005;169:569–576
Noordegraaf M, Flacher V, Stoitzner P, Clausen BE. Functional redundancy of Langerhans cells and Langerin+ dermal dendritic cells in contact hypersensitivity. J Invest Dermatol 2010;130:2752–2759
Kuipers H, Schnorfeil FM, Fehling HJ, Bartels H, Brocker T. Dicer-dependent microRNAs control maturation, function, and maintenance of Langerhans cells in vivo. J Immunol 2010;185:400–409
ahner SP, Kel JM, Martina CA, Brouwers-Haspels I, van Roon MA, Clausen BE. Conditional deletion of TGF-betaR1 using Langerin-Cre mice results in Langerhans cell deficiency and reduced contact hypersensitivity. J Immunol 2011;187:5069–5076
Kel JM, Girard-Madoux MJ, Reizis B, Clausen BE. TGF-beta is required to maintain the pool of immature Langerhans cells in the epidermis. J Immunol 2010;185:3248–3255
Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev 2010;234:120–141
Kautz-Neu K, Noordegraaf M, Dinges S, Bennett CL, John D, Clausen BE, et al. Langerhans cells are negative regulators of the anti-Leishmania response. J Exp Med 2011;208:885–891
Shklovskaya E, O’Sullivan BJ, Ng LG, Roediger B, Thomas R, Weninger W, et al. Langerhans cells are precommitted to immune tolerance induction. Proc Natl Acad Sci U S A 2011;108:18049–18054
Ritter U, Meissner A, Scheidig C, Korner H. CD8 alpha- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur J Immunol 2004;34:1542–1550
Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 2011;35:260–272
Kashem SW, Igyarto BZ, Gerami-Nejad M, Kumamoto Y, Mohammed J, Jarrett E, et al. Candida albicans Morphology and Dendritic Cell Subsets Determine T Helper Cell Differentiation. Immunity 2015;42:356–366
Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol 2014;14:417–428
Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med 2010;207:189–206
Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 2013;39:925–938
Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med 2007;204:3119–3131
Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med 2009;206:3115–3130
Shklovskaya E, Roediger B, Fazekas de St Groth B. Epidermal and dermal dendritic cells display differential activation and migratory behavior while sharing the ability to stimulate CD4(+) T cell proliferation in vivo. J Immunol 2008;181:418–430
Kumamoto Y, Linehan M, Weinstein JS, Laidlaw BJ, Craft JE, Iwasaki A. CD301b(+) dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 2013;39:733–743
Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, et al. Cross-presentation of viral and self antigens by skin-derived CD103(+) dendritic cells. Nat Immunol 2009;10:488–495
Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, et al. CD207(+) CD103(+) dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells (vol 207, pg 189, 2010). J Exp Med 2010;207:445–445
Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, et al. Identification of a novel population of Langerin(+) dendritic cells. J Exp Med 2007;204:3147–3156
Honda T, Nakajima S, Egawa G, Ogasawara K, Malissen B, Miyachi Y, et al. Compensatory role of Langerhans cells and langerin-positive dermal dendritic cells in the sensitization phase of murine contact hypersensitivity. J Allergy Clin Immunol 2010;125:1154–1156 e2
Murakami R, Denda-Nagai K, Hashimoto S, Nagai S, Hattori M, Irimura T. A Unique Dermal Dendritic Cell Subset That Skews the Immune Re-sponse toward Th2. PLoS One 2013;8:e73270.
Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polaizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol 2010;126:976–984, 984 e1–5
Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005;202:1213–1223
Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 2009;129:1339–1350
Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 2007;356:580–592
Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008;371:1665–1674
Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med 2007;204:3183–3194
Wohn C, Ober-Blobaum JL, Haak S, Pantelyushin S, Cheong C, Zahner SP, et al. Langerin(neg) conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc Natl Acad Sci U S A 2013;110:10723–10728
Yoshiki R, Kabashima K, Honda T, Nakamizo S, Sawada Y, Sugita K, et al. IL-23 from Langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A-producing gammadelta T cells. J Invest Dermatol 2014;134:1912–1921
Glitzner E, Korosec A, Brunner PM, Drobits B, Amberg N, Schonthaler HB, et al. Specific roles for dendritic cell subsets during initiation and progression of psoriasis. EMBO Mol Med 2014; 6: 1312–1327
Tortola L, Rosenwald E, Abel B, Blumberg H, Schafer M, Coyle AJ, et al. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest 2012;122:3965–3976
Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol 2005;23:901–944
Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001;2:675–680
Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol 2003;3:371–382
Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003; 3: 23–35
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122:787–795
Martinez FO, Helming L, Gordon S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Ann Rev of Immunol 2009;27:451–483
Kreider T, Anthony RM, Urban JF, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol 2007;19:448–453
Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011;11:723–737
Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989;74:2527–2534
Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, et al. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity 2013;38:570–580
Gordon EJ, Rao S, Pollard JW, Nutt SL, Lang RA, Harvey NL. Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development 2010;137:3899–3910
Abtin A, Jain R, Mitchell AJ, Roediger B, Brzoska AJ, Tikoo S, et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol 2014;15:45–53
Egawa G, Kabashima K. Skin as a Peripheral Lymphoid Organ: Revisiting the Concept of Skin-Associated Lymphoid Tissues. J Invest Dermatol 2011;131:2178–2185
Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol 2010;130:362–370
Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol 2004;4:211–222
Honda T, Egawa G, Grabbe S, Kabashima K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol 2013; 133: 303–315
Kim BS, Wojno ED, Artis D. Innate lymphoid cells and allergic inflammation. Curr Opin Immunol 2013;25:738–744
Kurebayashi Y, Nagai S, Ikejiri A, Koyasu S. Recent advances in understanding the molecular mechanisms of the development and function of Th17 cells. Genes Cells 2013;18:247–265
Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med 2014;20: 1043–1049
Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol 2009;9:316–321
Nomura T, Kabashima K, Miyachi Y. The panoply of alpha beta T cells in the skin. J Dermatol Sci 2014;76:3–9
Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T Cell Subsets, Migration Patterns, and Tissue Residence. Ann Rev Immunol Vol 31 2013; 31: 137–161
Schenkel JM, Masopust D. Tissue-Resident Memory T Cells. Immunity 2014;41:886–897
Brandtzaeg P, Pabst R. Let’s go mucosal: communication on slippery ground. Trends Immunol 2004;25:57–577
Toews GB, Bergstresser PR, Streilein JW. Langerhans cells: sentinels of skin associated lymphoid tissue. J Invest Dermatol 1980;75:78–82
Rubenfeld MR, Silverstone AE, Knowles DM, Halper JP, De Sostoa A, Fenoglio CM, et al. Induction of lymphocyte differentiation by epidermal cultures. J Invest Dermatol 1981;77:221–224
Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 2004;10:927–934
Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 2014;346:93–98
Girard-Madoux MJ, Kel JM, Reizis B, Clausen BE. IL-10 controls dendritic cell-induced T-cell reactivation in the skin to limit contact hypersensitivity. J Allergy Clin Immunol 2012;129:143–150 e1–10
Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and „Type 1“ inflammatory gene expression. Trends Immunol 2004;25:295–305
Johnson-Huang LM, Suarez-Farinas M, Pierson KC, Fuentes-Duculan J, Cueto I, Lentini T, et al. A single intradermal injection of IFN-gamma induces an inflammatory state in both non-lesional psoriatic and healthy skin. J Invest Dermatol 2012;132:1177–1187
Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol 2014;193: 3717–3725
Magro CM, Crowson AN, Kovatich AJ, Burns F. Lupus profundus, indeterminate lymphocytic lobular panniculitis and subcutaneous T-cell lymphoma: a spectrum of subcuticular T-cell lymphoid dyscrasia. J Cutan Pathol 2001;28:235–247
Massone C, Kodama K, Salmhofer W, Abe R, Shimizu H, Parodi A, et al. Lupus erythematosus panniculitis (lupus profundus): Clinical, histopathological, and molecular analysis of nine cases. J Cutan Pathol 2005;32:396–404
Bagabir R, Byers RJ, Chaudhry IH, Muller W, Paus R, Bayat A. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br J Dermatol 2012;167:1053–1066
Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol 1998;38:877–895
Lackey JN, Xia Y, Cho S, Sperling LC. Cutaneous lymphoid hyperplasia: a case report and brief review of the literature. Cutis 2007;79:445–448
Ono S, Kabashima K. Proposal of skin associated lymphoid tissue. Exp Dermatol 2015; 24:630–631
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflicts of interest
The authors declare that there are no conflicts of interest.
Cite this as
Ono S, Kabashima K. Novel insights into the role of immune cells in skin and inducible skin-associated lymphoid tissue (iSALT). Allergo J Int 2015; 24: 170–9 DOI: 10.1007/s40629-015-0065-1
Rights and permissions
About this article
Cite this article
Ono, S., Kabashima, K. Novel insights into the role of immune cells in skin and inducible skin-associated lymphoid tissue (iSALT). Allergo J 24, 18–27 (2015). https://doi.org/10.1007/s15007-015-0911-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15007-015-0911-y