Abstract
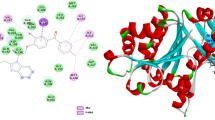
Antimicrobial activities of (−)-catechin derivatives were assayed for their antibacterial and antifungal activities against gram positive, gram negative bacteria, and fungi. Most of the compounds significantly active among which Compounds 1a, 1b, and 1c showed excellent antibacterial for both gram negative and gram positive bacteria, these compounds also exhibited excellent antifungal activity more than the standard drug. Molecular docking studies of Compounds 1a and 1b established good binding affinity with ATP-binding pocket of DNA gyrase and are in favor of the observed biological activity. These data collectively suggest that Compounds 1a and 1b could serve as a novel antimicrobial agent.





Similar content being viewed by others
References
Alanis AJ (2005) Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res 36:697–705
Amsterdam D (1996) Susceptibility testing of antimicrobials in liquid media. In: Loman V (ed) Antibiotics in laboratory medicine, 4th edn. Williams and Wilkins, Baltimore, pp 52–111
Chyu KY, Babbidge SM, Zhao X, Dandillaya R, Rietveld AG, Yano J, Dimayuga P, Cercek B, Shah PK (2004) Differential effects of green tea-derived catechin on developing versus established atherosclerosis in apolipoprotein E-null mice. Circulation 109:2448–2453
Fernandes R, Amador P, Prudencio C (2013) β-Lactams: chemical structure, mode of action and mechanisms of resistance. Rev Med Microbiol 24:7–17
Gadow AV, Joubert E, Hansmann CF (1997) Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong and black tea. Food Chem 60:73–77
Gradisar H, Pristovsek P, Plaper A, Jerala R (2007) Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J Med Chem 50:264–271
Graham HN (1992) Green tea composition, consumption, and polyphenol chemistry. Prev Med 21:334–350
Holdgate GA, Tunnicliffe A, Ward WH, Weston SA, Rosenbrock G, Barth PT, Taylor IW, Pauptit RA, Timms D (1997) The entropic penalty of ordered water accounts for weaker binding of the antibiotic novobiocin to a resistant mutant of DNA gyrase: a thermodynamic and crystallographic study. Biochemistry 36:9663–9673
Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS (2002) Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res 62:7241–7246
Hu ZQ, Zhao WH, Hara Y, Shimamura T (2001) Epigallocatechin gallate synergy with ampicillin-sulbactam against 28 clinical isolates of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 48:361–364
Husain A, Rashid M, Mishra R, Parveen S, Shin D-S, Kumar D (2012) Benzimidazole bearing oxadiazole and triazolo-thiadiazoles nucleus: design and synthesis as anticancer agents. Bioorg Med Chem Lett 22:5438–5444
Kumar D, Harshavardhan SJ, Chirumarry S, Poornachandra Y, Jang K, Kumar CG, Yoon YJ, Zhao BX, Miao JY, Shin D-S (2015) Design, synthesis in vitro anticancer activity and docking studies of (−)-catechin derivatives. Bull Korean Chem Soc 36:564–570
Miura Y, Chiba T, Tomita I, Koizumi H, Miura S, Umegaki K, Hara Y, Ikeda M, Tomita T (2001) Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr 131:27–32
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Park KD, Cho SJ (2010) Synthesis and antimicrobial activities of 3-O-alkyl analogues of (+)-catechin: improvement of stability and proposed action mechanism. Eur J Med Chem 45:1028–1033
Park KD, Lee SG, Kim SU, Kim SH, Sun WS, Cho SJ, Jung DH (2004) Anticancer activity of 3-O-acyl and alkyl-(−)-epicatechin derivatives. Bioorg Med Chem Lett 14:5189–5192
Paschka AG, Butler R, Young CY (1998) Induction of apoptosis in prostate cancer cell lines by the green tea component, (−)-epigallocatechin-3-gallate. Cancer Lett 130:1–7
Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg AL, Schacht SR (2010) How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov 9:203–214
Stapleton PD, Shah S, Anderson JC, Hara Y, Hamilton-Miller JM, Taylor PW (2004) Modulation of beta-lactam resistance in Staphylococcus aureus by catechins and gallates. Int J Antimicrob Agents 23:462–467
Uesato S, Kitagawa Y, Hara Y, Tokuda H, Okuda M, Mou XY, Nishino TH (2000) Antitumor promoting activities of 3-O-acyl-(−)epigallocatechins. Bioorg Med Chem Lett 10:1673–1675
Xie YS, Kumar D, Vijaykumar BVD, Tarani PS, Zhao BX, Jun YM, Jang K, Shin D-S (2014) Microwave-assisted parallel synthesis of benzofuran-2-carboxamide derivatives bearing anti-inflammatory, analgesic and antipyretic agents. Tetrahedron Lett 55:2796–2800
Acknowledgments
This work was supported by the Grants from Ministry of Environment (KME, 412-111-008) and National Research Foundation (NRF-2009-0094063), Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, D., Poornima, M., Kushwaha, R.N. et al. Antimicrobial and docking studies of (−)-catechin derivatives. J Korean Soc Appl Biol Chem 58, 581–585 (2015). https://doi.org/10.1007/s13765-015-0079-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-015-0079-x