Abstract
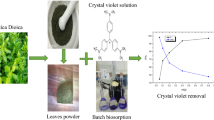
Butea monosperma leaf powder was used as an adsorbent to remove methylene blue dye from aqueous solution. Different parameters that affect on adsorption such as dye concentration, adsorbent dose, contact time and pH of the solution were analyzed. The suitable dye concentration was found to be 100 mg/l for adsorption experiments. The adsorbent dose and optimum contact time were standardized as 0.5 g/l and 120 min, respectively. The acceptable pH of the medium for adsorption was found as pH = 8. At the standardized environment, the maximum adsorption efficiency of the adsorbent was recorded as 98.70%. The ultra-morphological structure disclosed that the adsorbent had originally rugged surface with empty spaces on it, and after adsorption of the dye, these pores and spaces were filled up with the dye. Fourier transformed infrared spectroscopy chromatogram of the adsorbent shows many downward peaks which can be attributed to the existence of functional groups like C=O, –OH and –NH, etc. The suitably studied isotherm model was the Freundlich model having the correlation coefficient (r2) value of 0.9810. The adsorbent was transferred to the acidic environment (pH 6). At the pH, about 80% adsorbed dye was exuded. The adsorbent can be used for maximum two times for removal of the dye. Therefore, it can be concluded that Butea monosperma leaf powder has potential to remove methylene blue in aqueous solution. The absorbed dye can easily be recovered from adsorbent for reuse purposes.










Similar content being viewed by others
References
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112(10):5073–5091
Ali I, Aboul-Enein HY (2002) Speciation of arsenic and chromium metal ions by reversed phase high performance liquid chromatography. Chemosphere 48(3):275–278
Ali I, Aboul-Enein HY (2006) Instrumental methods in metal ion speciation, 1st edn. CRC Press, Boca Raton, pp 1–376
Ali I, Gupta VK (2006) Advances in water treatment by adsorption technology. Nat Protoc 1:2661–2667
Ali I, Khan TA, Asim M (2011) Removal of arsenic from water by electro coagulation and electro dialysis techniques. Sep Purif Rev 40:25–42. https://doi.org/10.1080/15422119.2011.542738
Ali I, Gupta VK, Khan TA, Asim M (2012a) Removal of arsenate from aqueous solution by electro coagulation method using Al–Fe electrodes. Int J Electrochem Sci 7:1898–1907
Ali I, Khan TA, Asim M (2012b) Removal of arsenate from ground water by electro coagulation method. Environ Sci Pollut Res 19(5):1668–1676
Ali I, AL-Othman ZA, Alwarthan AR et al (2014) Removal of arsenic species from water by batch and column operations on bagasse fly ash. Environ Sci Pollut Res 21:3218–3229. https://doi.org/10.1007/s11356-013-2235-3
Ali I, AL-Othman ZA, Sanagi MM (2015) Green synthesis of iron nano-impregnated adsorbent for fast removal of fluoride from water. J Mol Liq 211:457–465. https://doi.org/10.1016/j.molliq.2015.07.034
Ali I, AL-Othman ZA, Alharbi OML (2016a) Uptake of pantoprazole drug residue from water using novel synthesized composite iron nano adsorbent. J Mol Liq 218:465–472. https://doi.org/10.1016/j.molliq.2016.02.088
Ali I, AL-Othman ZA, Alwarthan AR (2016b) Synthesis of composite iron nano adsorbent and removal of ibuprofen drug residue from water. J Mol Liq 219:858–864. https://doi.org/10.1016/j.molliq.2016.04.031
Ali I, AL-Othman ZA, Al-Warthan A (2016c) Sorption, kinetics and thermodynamics studies of atrazine herbicide removal from water using iron nano composite material. Int J Environ Sci Technol 13:733. https://doi.org/10.1007/s13762-015-0919-6
Ali I, AL-Othman ZA, Alwarthan AR (2016d) Green synthesis of functionalized iron nano particles and molecular liquid phase adsorption of ametryn from water. J Mol Liq 221:1168–1174
Ali I, AL-Othman ZA, Alwarthan AR (2016e) Molecular uptake of congo red dye from water on iron composite nano particles. J Mol Liq 224:171–176
Ali I, Alharbi Omar OML, Alothman ZA, Badjah AY, Alwarthan AR, Basheer AA (2018) Artificial neural network modelling of amido black dye sorption on iron composite nano material: kinetics and thermodynamics studies. J Mol Liq 250:1–8. https://doi.org/10.1016/j.molliq.2017.11.163
Arami M, Limaee NY, Mahmoodi NM, Tabrizi NS (2006) Equilibrium and kinetics for the adsorption of direct acid dyes from aqueous solution by soy meal hull. J Hazard Mater 135:171–179
Banat F, Al-Asheh S, Al-Makhadmeh L (2003) Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process Biochem 39:193–202
Basheer AA (2018) Chemical chiral pollution: impact on the society and science and need of the regulations in the twenty-first century. Chirality 30(4):402–406. https://doi.org/10.1002/chir.22808
Bulut Y, Aydin H (2006) A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 194:259–267
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265:159–168
Dural MU, Cavas L, Papageorgiou SK, Katsaros FK (2011) Methylene blue adsorption on activated carbon prepared from Posidonia oceanica (L.) dead leaves: kinetics and equilibrium studies. Chem Eng J 168:77–85
Fuks L, Herdzik-Koniecko I, Maskalchuk L, Leontieva T (2018) Clay-salt slimes of the JSC “Belaruskali” as potential engineering barriers in the radioactive waste repositories: sorption of Cs(I), Sr(II), Eu(III) and Am(III). Int J Environ Sci Technol 15:2047–2058
Gupta VK, Ali I (2012) Environmental water—advances in treatment, remediation and recycling, vol 1, 1st edn. Elsevier, Amsterdam, p 232
Gupta N, Kushwaha AK, Chattopadhyaya MC (2016) Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arab J Chem 9(1):S707–S716. https://doi.org/10.1016/j.arabjc.2011.07.021
Hameed BH (2009) Removal of cationic dye from aqueous solution using jackfruit peel as non-conventional low-cost adsorbent. J Hazard Mater 162:344–350
Hameed BH (2011) Grass waste: a novel sorbent for the removal of basic dye from aqueous solution. Desalination 265:159–168
Hameed BH, El-Khaiary MI (2008) Batch removal of malachite green from aqueous solutions by adsorption on oil palm trunk fibre: equilibrium isotherms and kinetic studies. J Hazard Mater 154:237–244
Hameed BH, Mahmoud DK, Ahmad AL (2008) Sorption equilibrium and kinetics of basic dye from aqueous solution using banana stalk waste. J Hazard Mater 158:499–506
Malik PK (2003) Use of activated carbons prepared from sawdust and rice-husk for adsoprtion of acid dyes: a case study of acid yellow. Dyes Pigm 56:239–249
Mishra M, Shukla YN, Kumar S (2000) Euphane triterpenoid and lipid constituents from Butea monosperma. Phytochemistry 54:835–848
Mishra Y, Sowmya V, Shanthakumar S (2015) Adsorption studies of basic dyes onto Teak (Tectona grandis) leaf powder. J Urban Environ Eng 9:102–108
Nasuha N, Hameed BH, Mohddin AT (2010) Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J Hazard Mater 175:126–132
Nethaji S, Sivasamy A, Thennarasu G, Saravanan S (2010) Adsorption of Malachite Green dye onto activated carbon derived from Borassus aethiopum flower biomass. J Hazard Mater 181:271–280
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Sehrawat A, Kumar V (2012) Butein imparts free radical scavenging, anti-oxidative and pro apoptotic properties in the flower extracts of Butea monosperma. Biocell 36:63–71
Senthilkumaar S, Varadarajan PR, Porkodi K, Subbhuraam CV (2005) Adsorption of methylene blue onto jute fiber carbon: kinetics and equilibrium studies. J Colloid Interface Sci 284:78–82
Sharma A, Bhattacharyya KG (2005) Utilization of a biosorbent based on Azadirachta indica neem leaves for removal of water-soluble dyes. Indian J Chem Technol 12:285–295
Sivaraj R, Namasivayam C, Kadirvelu K (2001) Orange peels an adsorbent in the removal of acid violet 17 (acid dye) from aqueous solutions. Waste Manag 21:105–110
Song J, Zou W, Bian Y, Su F, Han R (2011) Adsorption characteristics of methylene blue by peanut husk in batch and column modes. Desalination 265:119–125
Sumitra M, Manikandan P, Suguna L (2005) Efficacy of Butea monosperma on dermal wound healing in rats. Int J Biochem Cell Biol 37(3):566–573
Uddin MT, Rukanuzzaman M, Khan MMR, Islam MA (2009) Jackfruit (Artocarpus heterophyllus) leaf powder: an effective adsorbent for removal of methylene blue from aqueous solutions. Indian J Chem Technol 16:142–149
Vieira AP, Santana SAA, Bezerra CWB, Silva HAS, Chaves JAP, Melo JCP, Filho ECS, Airoldi C (2009) Kinetics and thermodynamics of textile dye adsorption from aqueous solutions using Babassu coconut mesocarp. J Hazard Mater 166:1272–1278
Wenga CH, Linb YT, Tzeng TW (2009) Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. J Hazard Mater 170:417–424
Acknowledgements
The authors wish to thank management of GIET University, Gunupur, for providing laboratory facilities to carry out the investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest
Additional information
Editorial responsibility: Necip Atar.
Rights and permissions
About this article
Cite this article
Das, M., Samal, A.K. & Mehar, N. Butea monosperma leaf as an adsorbent of methylene blue: recovery of the dye and reuse of the adsorbent. Int. J. Environ. Sci. Technol. 17, 2105–2112 (2020). https://doi.org/10.1007/s13762-019-02480-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02480-7