Abstract
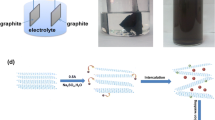
Graphene oxide nanosheets were synthesized by electrochemical exfoliation. X-ray diffraction, scanning electron microscopy, atomic force microscopy, Raman spectrometry and Fourier transform infrared spectrometry were used to characterize crystal structure, particle size, thickness and function groups of the nanosheets. The nanosheets were examined for adsorption of methyl orange, an anionic dye, in aqueous solution at different pHs and temperatures. The maximum adsorption capacity of methyl orange on graphene oxide nanosheets obtained from the Langmuir isotherm was 138.69 mg/g at pH 2.0, which is larger than that of other carbonaceous adsorbents. The large adsorption affinity of graphene oxide nanosheets to methyl orange might be due to the presence of hydrogen bonding and π–π interaction between methyl orange and graphene oxide nanosheets. Adsorption kinetics followed a pseudo-second-order kinetic model, and the isotherm adsorption results were fitted with Langmuir isotherm model in a monolayer adsorption manner. The thermodynamic studies indicated that the adsorption reaction was a spontaneous physisorption process.






Similar content being viewed by others
References
Akhlaghian F, Ghadermazi M, Chenarani B (2014) Removal of phenolic compounds by adsorption on nano structured aluminosilicates. J Environ Chem Eng 2:543–549
Al-Bastaki N (2004) Removal of methyl orange dye and Na2SO4 salt from synthetic waste water using reverse osmosis. Chem Eng Proc 43:1561–1567
Elwakeel KZ, Guibal E (2015) Selective removal of Hg(II) from aqueous solution by functionalized magnetic-macromolecular hybrid material. Chem Eng J 281:345–359
Elwakeel KZ, El-Bindary AA, El-Sonbati AZ, Hawas AR (2016) Adsorption of toxic acidic dye from aqueous solution onto diethylenetriamine functionalized magnetic glycidyl methacrylate-N,N′-methylenebisacrylamide. RSC Adv 6:3350–3361
Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, Mauri F, Piscanec S, Jiang D, Novoselov KS, Roth S, Germ AK (2006) Raman spectrum of graphene and graphene layers. Phys Rev Lett 97:187401–187405
Freundlich H (1906) Concerning adsorption in solutions. J Phys Electrochem 57:385–470
Graf D, Molitor F, Ensslin K, Stampfer C, Jungen A, Hierold C, Wirtz L (2007) Spatially resolved raman spectroscopy of single- and few-layer graphene. Nano Lett 7:238–242
Gu L, Zhu NW, Guo HQ, Huang SQ, Lou ZY, Yuan HP (2013) Adsorption and fenton-like degradation of naphthalene dye intermediate on sewage sludge derived porous carbon. J Hazard Mater 246:145–153
Gupta A, Chen G, Joshi P, Tadigadapa S, Eklund PC (2006) Raman scattering from high-frequency phonons in supported n-graphene layer films. Nano Lett 6:2667–2673
Han F, Kambala VSR, Srinivasan M, Rajarathnam D, Naidu R (2009) Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: a review. Appl Catal A Gen 359:25–40
Ho YS, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss flat. Water Res 34:735–742
Huang JH, Huang KL, Liu Q, Wang AT, Yan C (2008) Adsorption of rhodamine B and methyl orange on a hypercrosslinked polymeric adsorbent in aqueous solution. Colloids Surf A: Physicochem Eng Aspects 330:55–61
Huber P, Carre B (2012) Decolorization of process waters in drinking mills and similar applications: a review. Bioresources 7:1366–1382
Konicki W, Cendrowski K, Chen XC, Mijowska E (2013) Application of hollow mesoporous carbon nanospheres as a high effective adsorbent for the fast removal of acid dyes from aqueous solutions. Chem Eng J 228:824–833
Kornaros M, Lyberatos G (2006) Biological treatment of wastewaters from dye manufacturing company using a trickling filter. J Hazard Mater 136:95–102
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I Solids J Am Chem Soc 38:2221–2295
Latil S, Henrard L (2006) Charge carriers in few-layer graphene films. Phys Rev Lett 97:036803–036806
Lee C, Wei X, Kysar JW, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321:385–388
Li X, Cai W, An J, Kim S, Nah J, Yang D, Piner R, Velamakanni A, Jung I, Tutuc E, Banerjee SK, Colombo L, Ruoff RS (2009) Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324:1312–1314
Liu N, Luo F, Wu H, Liu YH, Zhang C, Chen J (2009) One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv Funct Mater 18:1518–1525
Lu J, Yang JX, Wang JZ, Lim A, Wang S, Loh KP (2009) One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 3:2367–2375
Luo Z, Lu Y, Somers LA, Johnson ATC (2009) High yield preparation of macroscopic graphene oxide membranes. J Am Chem Soc 131:898–899
Mall ID, Srivastava VC, Agarwal NK (2006) Removal of Orange-G and methyl violet dyes by adsorption onto bagasse fly ash—kinetic study and equilibrium isotherm analyses. Dyes Pigments 69:210–223
Meyer JC, Geim AK, Katsnelson MI, Novoselov KS, BoothTJ Roth S (2007) The structure of suspended graphene sheets. Nature 446:60–63
Neves CMSS, Lemus J, Freire MG, Palomar J, Coutinho JAP (2014) Enhancing the adsorption of ionic liquids onto activated carbon by the addition of inorganic salts. Chem Eng J 252:305–310
Ni ZM, Xia SJ, Wang LG, Xing FF, Pan GX (2007) Treatment of methyl orange by calcined layered double hydroxides in aqueous solution: adsorption property and kinetic studies. J Colloid Interface Sci 316:284–291
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Park S, Ruoff RS (2009) Chemical Methods for the production of graphenes. Nat Nanotechnol 4:217–224
Ramesha GK, Kumara AV, Muralidhara HB, Sampath S (2011) Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J Colloid Interface Sci 361:270–277
Reina A, Jia X, Ho J, Nezich D, Son H, Bulovic V, Dresselhaus MS, Kong J (2009) Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett 9:30–35
Sandberg RG, Henderson GH, White RD, Eyring EM (1972) Kinetics of acid dissociation-ion recombination of aqueous methyl orange. J Phys Chem 76:4023–4025
Sharma P, Hussain N, Borah DJ, Das MR (2013) Kinetics and adsorption behavior of the methyl blue at the graphene oxide/reduced graphene oxide nanosheet–water interface: a comparative study. J Chem Eng Data 58:151–158
Singh KP, Mohan D, Sinha S, Tondon GS, Gosh D (2003) Color removal from wastewater using low-cost activated carbon derived from agricultural waste material (Gosh, D). Ind Eng Chem Res 42:1965–1976
Singh VV, Cupta G, Batra A, Nigam AK, Boopathi M, Gutch PK, Tripathi BK, Srivastava A, Samuel M, Agarwal GS, Singh B, Vijayaraghavan R (2012) Greener electrochemical synthesis of high quality graphene nanosheets directly from pencil and its SPR sensing application. Adv Funct Mater 22:2352–2362
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Su C-Y, Lu A-Y, Xu Y, Chen F-R, Khlobystov AN, Li LJ (2011) High-quality thin graphene films from fast electrochemical exfoliation. ACS Nano 5(3):2332–2339
Travlou NA, Kyzas GZ, Lazaridis NK, Deliyanni EA (2013) Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir 29:1657–1668
Vecitis CD, Gao G, Liu H (2011) Electrochemical Carbon nanotube filter for adsorption, desorption, and oxidation of aqueous dyes and anions. J Phys Chem C 115:3621–3629
Wang S, Yu DM, Huang Y, Guo JC (2011) The adsorption of sulphonated azo-dyes methyl orange and xylenol orange by coagulation on hollow chitosan microsphere. J Appl Poly Sci 119:2065–2071
Wu T, Cai X, Tan S, Li H, Liu J, Yang W (2011) Adsorption characteristics of acrylonitrile, p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions. Chem Eng J 173:144–149
Yao YJ, He B, Xu FF, Chen XF (2011) Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes. Chem Eng J 170:82–89
Acknowledgments
This work was financially supported by the National Basic Research Program of China (Grant Number: 2011CB936003), National Natural Science Foundation of China (Grant Number: 21373183) and Zhejiang Provincial Natural Science Foundation of China (Grant Number: LY12B07001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Gong, J., Gao, X., Li, M. et al. Dye adsorption on electrochemical exfoliated graphene oxide nanosheets: pH influence, kinetics and equilibrium in aqueous solution. Int. J. Environ. Sci. Technol. 14, 305–314 (2017). https://doi.org/10.1007/s13762-016-1143-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1143-8