Abstract
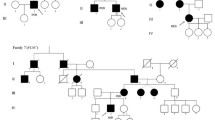
McArdle disease (MD) is a metabolic myopathy caused by deficiency of the myophosphorylase enzyme. The aim of our study was to analyse a series of MD patients in Brazil and the correlation between clinical findings, laboratory data, electromyography, muscle biopsy and genetic features. The PYGM gene was analysed by PCR/RLFP and Sanger sequencing. The sample included 12 patients, aged 18–57 years, from unrelated families. Exercise intolerance was present in all cases. Serum creatine kinase levels at rest were increased in all patients. Forearm ischaemic exercise testing in five patients revealed no increase in venous lactate. Needle electromyography presented ‘myopathic pattern’ in six patients. Muscle biopsy showed vacuolar myopathy in 10 patients and deficiency of myophosphorylase enzyme in all patients. The genetic analysis showed p.R50X as the most common mutation (allelic frequency: 56.25%), other known mutations (p.Y574X, p.G205S, p.W798R, IVS14 + 1G > A and IVS19-1G > A) and a new mutation (p.Asn168Lysfs*15) were also identified. Several features of the disorder were similar to the vast majority of patients worldwide. The genetic findings of this study revealed a range of mutations that are quite similar to the European cohort. The discovery of one novel mutation increases the genotypic heterogeneity of PYGM gene.



Similar content being viewed by others
References
Fernandez R, Navarro C, Andreu AL, Bruno C, Shanske S, Gamez J et al (2000) A novel missense mutation (W797R) in the myophosphorylase gene in Spanish patients with McArdle disease. Arch Neurol 57:217–219
Santalla A, Nogales-Gadea G, Encinar AB, Vieitez I, Gonzalez-Quintana A, Serrano-Lorenzo P et al (2017) Genotypic and phenotypic features of all Spanish patients with McArdle disease: a 2016 update. BMC Genom 18(Suppl 8):819
De Castro M, Johnston J, Biesecker L (2015) Determining the prevalence of McArdle disease from gene frequency by analysis of next-generation sequencing data. Genet Med 17:1002–1006
Vieitez I, Teijeira S, Fernandez JM, San Millan B, Miranda S, Ortolano S et al (2011) Molecular and clinical study of McArdle’s disease in a cohort of 123 European patients. Identification of 20 novel mutations. Neuromuscul Disord 21:817–823
Martinuzzi A, Tsujino S, Vergani L, Schievano G, Cadaldini M, Bartoloni L et al (1996) Molecular characterization of myophosphorylase deficiency in a group of patients from northern Italy. J Neurol Sci 137:14–19
Deschauer M, Morgenroth A, Joshi PR, Glaser D, Chinnery PF, Aasly J et al (2007) Analysis of spectrum and frequencies of mutations in McArdle disease. Identification of 13 novel mutations. J Neurol 254:797–802
Scalco RS, Morrow JM, Booth S, Chatfield S, Godfrey R, Quinlivan R (2017) Misdiagnosis is an important factor for diagnostic delay in McArdle disease. Neuromusc Dis 27:852–855
Kubisch C, Wicklein EM, Jentsch TJ (1998) Molecular diagnosis of McArdle disease: revised genomic structure of the myophosphorylase gene and identification of a novel mutation. Hum Mutat 12:27–32
Quinlivan R, Andreu AL, Marti R (2017) 211th ENMC international workshop: development of diagnostic criteria and management strategies for McArdle disease and related rare glycogenolytic disorders to improve standards of care 17–19th April 2015 Naarden, Netherlands. Neuromuscul Disord 27:1143–1151
Martin MA, Rubio JC, Buchbinder J, Fernandez-Hojas R, del Hoyo P, Teijeira S et al (2001) Molecular heterogeneity of myophosphorylase deficiency (McArdle’s disease): a genotype-phenotype correlation study. Ann Neurol 50:574–581
Inal-Gültekin G, Toptas-Hekimoglu B, Görmez Z, Gelisin O, Durmus H, Erquner B et al (2017) Myophosphorylase (PYGM) mutations determined by next generation sequencing in a cohort from Turkey with McArdle disease. Neuromusc Dis 27:997–1008
Lorenzoni PJ, Silvado CE, Scola RH, Luvizotto M, Werneck LC (2007) McArdle disease with rhabdomyolysis induced by rosuvastatin: case report. Arq Neuropsiquiatr 65:834–837
Lorenzoni PJ, Lange MC, Kay CS, Scola RH, Werneck LC (2005) Motor nerve conduction study in McArdle disease: case report. Arq Neuropsiquiatr 63:874–877
Gurgel-Giannetti J, Nogales-Gadea G, van der Linden H Jr, Bellard TM, Brasileiro Filho G, Giannetti AV et al (2013) Clinical and molecular characterization of McArdle’s disease in Brazilian patients. Neuromol Med 15:470–475
Zutt R, van der Kooi AJ, Linthorst GE, Wanders RJA, Visser M (2014) Rhabdomyolysis: review of the literature. Neuromusc Dis 24:651–659
Martinuzzi A, Sartori E, Fanin M, Nascimbeni A, Valente L, Angelini C et al (2003) Phenotype modulators in myophosphorylase deficiency. Ann Neurol 53:497–502
Werneck LC (1981) The value of muscle biopsy in neurology: a study of 290 biopsies. Rev Bras Clin Ter 10(Suppl):2–24
Andreu AL, Nogales-Gadea G, Cassandrini D, Arenas J, Bruno C (2007) McArdle disease: molecular genetic update. Acta Myol 26:53–57
García-Consuegra I, Asensio-Peña S, Ballester-Lopez A, Francisco-Velilla R, Pinos T, Pintos-Morell G et al (2018) Missense mutations have unexpected consequences: the McArdle disease paradigm. Hum Mutat 2018 39:1338–1343
Tsujino S, Shanske S, DiMauro S (1993) Molecular genetic heterogeneity of myophosphorylase deficiency (McArdle’s disease). N Engl J Med 329:241–245
Hogrel JY, van den Bogaart F, Ledoux I, Ollivier G, Petit F, Koujah N et al (2015) Diagnostic power of the non-ischaemic forearm exercise test in detecting glycogenosis type V. Eur J Neurol 22:933–940
Kazemi-Esfarjani P, Skomorowska E, Jensen TD, Haller RG, Vissing J (2002) A nonischemic forearm exercise test for McArdle disease. Ann Neurol 52:153–159
Lindner A, Reichert N, Eichhorn M, Zierz S (2001) Acute compartment syndrome after forearm ischemic work test in a patient with McArdle’s disease. Neurology 56:1779–1780
Henning F, Cunninghame CA, Martin MA, Rubio JC, Arenas J, Lucia A et al (2017) Muscle fiber type proportion and size is not altered in McArdle disease. Muscle Nerve 55:916–917
Nogales-Gadea G, Brull A, Santalla A, Andreu AL, Arenas J, Martin MA et al (2015) McArdle disease: update of reported mutations and polymorphisms in the PYGM gene. Hum Mutat 36:669–678
Garcia-Consuegra I, Blázquez A, Rubio JC, Arenas J, Ballester-Lopez A, González-Quintana A et al (2016) Taking advantage of an old concept, “illegitimate transcription”, for a proposed novel method of genetic diagnosis of McArdle disease. Genet Med 18:1128–1135
Garcia-Consuegra I, Rubio JC, Nogales-Gadea G, Bautista J, Jimenez S, Cabello A et al (2009) Novel mutations in patients with McArdle disease by analysis of skeletal muscle mRNA. J Med Genet 46:198–202
Bruno C, Cassandrini D, Martinuzzi A, Toscano A, Moggio M, Morandi L et al (2006) McArdle disease: the mutation spectrum of PYGM in a large Italian cohort. Hum Mutat 27:718
Gámez J, Rubio JC, Martín MA, Fernandez-Cadenas I, Garcia-Arumi E, Anreu AL et al (2003) Two novel mutations in the muscle glycogen phosphorylase gene in McArdle’s disease. Muscle Nerve 28:380–382
Rubio JC, Martín MA, Campos Y, Auciello R, Cabello A, Arenas J (2000) A missense mutation W797R in the myophosphorylase gene in a Spanish patient with McArdle’s disease. Muscle Nerve 23:129–131
Tsujino S, Shanske S, Nonaka I, Eto Y, Mendell JR, Fenichel GM et al (1994) Three new mutations in patients with myophosphorylase deficiency (McArdle disease). Am J Hum Genet 54:44–52
Moro A, Munhoz RP, Arruda WO, Raskin S, Moscovich M, Teive HA (2014) Spinocerebellar ataxia type 3: subphenotypes in a cohort of Brazilian patients. Arq Neuropsiquiatr 72:659–662
Mihaylova V, Scola RH, Gervini B, Lorenzoni PJ, Kay CK, Werneck LC et al (2010) Molecular characterisation of congenital myasthenic syndromes in Southern Brazil. J Neurol Neurosurg Psychiatry 81:973–977
Cruz MW (2012) Regional differences and similarities of familial amyloidotic polyneuropathy (FAP) presentation in Brazil. Amyloid Suppl 1:65–67
Funding
This study was supported by UFPR, Fundação Araucária, CAPES and CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the Local Ethics Committee for Human Research.
Informed consent
All studies were conducted in accordance with ethical principles after obtaining patient consent.
Rights and permissions
About this article
Cite this article
Lorenzoni, P.J., Werneck, L.C., Kay, C.S.K. et al. Single-centre experience on genotypic and phenotypic features of southern Brazilian patients with McArdle disease. Acta Neurol Belg 120, 303–311 (2020). https://doi.org/10.1007/s13760-018-1038-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-018-1038-1