Abstract
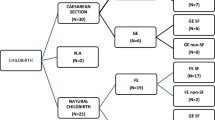
The aim of this study is to evaluate the effect of levetiracetam treatment during pregnancy on fetus.. The pregnant women with epilepsy (PWWE) who were exposed to levetiracetam treatment during pregnancy in the form of monotherapy or polytherapy were retrospectively evaluated. They were compared with the PWWE who did not use the antiepileptic drug (AED) during pregnancy. A total of 102 pregnancies were examined. While 35 patients never used AED during pregnancy, 30 patients received only levetiracetam therapy, and 37 patients received levetiracetam with at least one combined AED. While no major congenital malformation (MCM) was determined in the group of patients who never used AED and who received levetiracetam monotherapy, 2 MCMs were determined in the group receiving multiple AED therapy with levetiracetam. This study showed that the use of levetiracetam as monotherapy during pregnancy was at the same risk level as the group who never used AED and that the risk increased when it was used as a part of polytherapy. In conclusion, these findings support the current understanding that LEV may be a feasible option for PWWE.
Similar content being viewed by others
References
Chaudhry SA, Jong G, Koren G (2014) The fetal safety of Levetiracetam: a systematic review. Reprod Toxicol 46:40–45. https://doi.org/10.1016/j.reprotox.2014.02.004
Mawhinney E, Craig J, Morrow J, Russell A, Smithson WH, Parsons L, Morrison PJ, Liggan B, Irwin B, Delanty N, Hunt SJ (2013) Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology 80(4):400–405. https://doi.org/10.1212/WNL.0b013e31827f0874
ten Berg K, Samrén EB, van Oppen AC, Engelsman M, Lindhout D (2005) Levetiracetam use and pregnancy outcome. Reprod Toxicol 20(1):175–178
Hernández-Díaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, Yerby M, Holmes LB, North American AED Pregnancy Registry; North American AED Pregnancy Registry (2012) Comparative safety of antiepileptic drugs during pregnancy. Neurology 78(21):1692–1699. https://doi.org/10.1212/WNL.0b013e3182574f39
Gambardella A, Labate A, Colosimo E, Ambrosio R, Quattrone A (2008) Monotherapy for partial epilepsy: focus on levetiracetam. Neuropsychiatr Dis Treat 4(1):33–38
Reimers A, Brodtkorb E (2012) Second-generation antiepileptic drugs and pregnancy: a guide for clinicians. Expert Rev Neurother 12(6):707–717. https://doi.org/10.1586/ern.12.32
Hovinga CA (2001) Levetiracetam: a novel antiepileptic drug. Pharmacotherapy 21(11):1375–1388
Patsalos PN (2004) Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet 43(11):707–724
Vajda FJ, Graham J, Roten A, Lander CM, O’Brien TJ, Eadie M (2012) Teratogenicity of the newer antiepileptic drugs—the Australian experience. J Clin Neurosci 19(1):57–59. https://doi.org/10.1016/j.jocn.2011.08.003
Würtz AM, Rytter D, Vestergaard CH, Christensen J, Vestergaard M, Bech BH (2017) Prenatal exposure to antiepileptic drugs and use of primary healthcare during childhood: a population-based cohort study in Denmark. BMJ Open 7(1):e012836. https://doi.org/10.1136/bmjopen-2016-012836
Mølgaard-Nielsen D, Hviid A (2011) Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA 305(19):1996–2002. https://doi.org/10.1001/jama.2011.624
EUROCAT (2005) EUROCAT Guide 1.3 and reference documents, instructions for the registration and surveillance of congenital anomalies. http://www.eurocat.ulster.ac.uk/pdf/EUROCAT-Guide-1.3.pdf. Accessed 23 Dec 2017
Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, Irwin B, McGivern RC, Morrison PJ, Craig J (2006) Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 77(2):193–198. https://doi.org/10.1136/jnnp.2005.074203
Martinez Ferri M, Peña Mayor P, Perez López-Fraile I, Escartin Siquier A, Martin Moro M, Forcadas Berdusan M, en representación del registro EURAP España (2016) Comparative study of antiepileptic drug use during pregnancy over a period of 12 years in Spain. Efficacy of the newer antiepileptic drugs lamotrigine, levetiracetam, and oxcarbazepine. Neurologia. https://doi.org/10.1016/j.nrl.2016.05.004
Hunt S, Craig J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, Irwin B, Morrison PJ, Morrow J (2006) Levetiracetam in pregnancy: preliminary experience from the UK Epilepsy and Pregnancy Register. Neurology 67(10):1876–1879
Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, Perucca E, Vajda F, EURAP study group (2011) Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol 10:609–617. https://doi.org/10.1016/S1474-4422(11)70107-7
Battino D, Tomson T, Bonizzoni E, Craig J, Lindhout D, Sabers A, Perucca E, Vajda F, EURAP Study Group (2013) Seizure control and treatment changes in pregnancy: observations from the EURAP epilepsy pregnancy registry. Epilepsia 54(9):1621–1627. https://doi.org/10.1111/epi.12302
Schachter SC (2018) Risks associated with epilepsy and pregnancy. UpToDate. https://www.uptodate.com/contents/risks-associated-with-epilepsy-and-pregnancy. Acsessed 02 Feb 2018
Richmond JR, Krishnamoorthy P, Andermann E, Benjamin A (2004) Epilepsy and pregnancy: an obstetric perspective. Am J Obstet Gynecol 190(2):371–379
Vajda FJ, Hitchcock AA, Graham J, O’Brien TJ, Lander CM, Eadie MJ (2010) The teratogenic risk of antiepileptic drug polytherapy. Epilepsia 51(5):805–810. https://doi.org/10.1111/j.1528-1167.2009.02336.x
Vajda FJ, O’Brien T, Lander C, Graham J, Eadie M (2014) The efficacy of the newer antiepileptic drugs in controlling seizures in pregnancy. Epilepsia 55(8):1229–1234. https://doi.org/10.1111/epi.12711
Pirie DA, Al Wattar BH, Pirie AM, Houston V, Siddiqua A, Doug M, Bagary M, Greenhill L, Khan KS, McCorry D, Thangaratinam S (2014) Effects of monitoring strategies on seizures in pregnant women on lamotrigine: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 172:26–31. https://doi.org/10.1016/j.ejogrb.2013.10.021
Reisinger TL, Newman M, Loring DW, Pennell PB, Meador KJ (2013) Antiepileptic drug clearance and seizure frequency during pregnancy in women with epilepsy. Epilepsy Behav 29(1):13–18. https://doi.org/10.1016/j.yebeh.2013.06.026
Thomas SV, Syam U, Devi JS (2012) Predictors of seizures during pregnancy in women with epilepsy. Epilepsia 53(5):e85–e88. https://doi.org/10.1111/j.1528-1167.2012.03439.x
Harden CL, Hopp J, Ting TY, Pennell PB, French JA, Allen Hauser W, Wiebe S, Gronseth GS, Thurman D, Meador KJ, Koppel BS, Kaplan PW, Robinson JN, Gidal B, Hovinga CA, Wilner AN, Vazquez B, Holmes L, Krumholz A, Finnell R, Le Guen C, American Academy of Neurology; American Epilepsy Society (2009) Management issues for women with epilepsy-Focus on pregnancy (an evidence-based review): I. Obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia 50(5):1229–1236. https://doi.org/10.1111/j.1528-1167.2009.02128.x
Funding
There is no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Koc, G., Keskin Guler, S., Karadas, O. et al. Fetal safety of levetiracetam use during pregnancy. Acta Neurol Belg 118, 503–508 (2018). https://doi.org/10.1007/s13760-018-0996-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-018-0996-7