Abstract
Crystal violet (CV) synthetic dyes are well known in the dyeing industry for their mitotic and mutagenic poisoning. CV dye being a toxic organic dye is responsible for serious health issues as well as environmental damage. In this study, an inexpensive biosorbent (white clover: Trifolium repens) stem powder was tested for the adsorption of CV dye (cationic dye) from an aqueous solution. The batch adsorption measurements were designed to find out the influence of contact time, pH, adsorbent dose, and dye concentration, for dye removal. The operation parameters studied are the contact time (20 to 160 min), initial dye concentration (10–100 mg/L), dose (0.1–1 g), and pH, (1–10). At optimum conditions, maximum percent removal of 92.997% and adsorption capacity value of 1.952 mg/g was achieved at pH 2, adsorbent dose (1 g), and contact time (140 min), and dye concentration (70 ppm). The results suggested that the removal of CV rose with the contact time and adsorbent dose. Langmuir and Freundlich isotherm models were applied to the equilibrium adsorption data, and data were perfectly fitted to Langmuir isotherm model. Pseudo-first-order and pseudo-second-orders were applied to the data, and it was found that the pseudo-second-order kinetic model was best fitted to the experimental data. It was also revealed that the stem powdered of the Trifolium repens plant can be employed as a useful adsorbent to remove cationic CV dye from different water samples (tap, river and distilled). Furthermore, the performance of adsorbent was also evaluated in saline water containing sodium chloride, potassium chloride and manganese chloride salts to check the effect of various ions on the performance of adsorbent and it was observed that the adsorbent showed excellent performance in saline water. Moreover, a comparative study was performed to check the efficiency of different commercial adsorbents (silica gel and active carbon) and to compare their performance with our study. All these experiments revealed that the biosorbent used in this study effectively removes contaminating dyes from industrial wastewater and as well as saline water and thus, can be used for the treatment of wastewater at the commercial level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life could not have existed without the presence of water [27, 28]. Nowadays, water pollution is among the critical issues in the world due to its importance for life. Due to rapid industrialization, anthropogenic activities, unplanned urban migration, unsustainable usage of water resources, and rapid population growth, toxic pollutants are released into the atmosphere [16, 22, 24, 26]. Several industries, particularly paper and pulp industries, and textile industries, usually employ synthetic dyes and pigments for coloring their products [30]. Due to which 40,000–50,000 tons of dyes are being released into the water systems every year [15, 36]. These dyes reduce the aesthetic value of water bodies and interfere with light penetration, and, thus, damage the aquatic ecology [10]. Certain dyes and their byproducts have poisonous, mutagenic effects, and can cause cancer. These dyes also affect the kidney, liver, and neurological system malfunction in humans. The complicated structure of them makes their decolorization challenging. Thus, the color removal from wastewater is an important issue for environmentalists and researchers.
To reduce the influence of synthetic dyes on the environment, a variety of technologies were developed for their removal from wastewater. Chemical coagulation, oxidation, ozonation, flocculation, membrane-based separation techniques, biological processes, sonochemical processes, photocatalytic processes, adsorption electrochemical processes are just a few of the techniques used to remove colored dyes [4, 13, 14, 23, 41]. Among these physical–chemical techniques, electrochemical coagulation and adsorption are important and are used most commonly [11]. The adsorption techniques have attracted a great attention owing to their simplicity to use and flexibility. It is a simple and effective method that allows kinetic and equilibrium measurements without the need for highly complex equipment [12]. Although the usage of activated carbon as an adsorbent for the adsorption of basic dyes has been studied widely, its expensive cost prevents it from being used commercially. Several studies have been carried out recently to create new, affordable adsorbents. Many industrial and agricultural wastes, including waste apricots, sewage sludge, teak leaves, rice husk, pine wood sawdust, hardwood sawdust, coir pith, waste materials, orange peel, waste tires, hen feathers, deoiled soya and bottom ash, have been employed for the remove of dyes wastewater [27, 28].
In this study, an untreated form of absorbent (white clover: Trifolium repens) stem powder was employed to remove crystal violet (CV) dye. CV dye, a triphenylmethane group member, is largely employed in veterinary and animal medicine as a biological stain, to determine the bloody fingerprints, and in a variety of industrial textile processes [19]. CV is a stable dye and is resistant to microbial degradation. Therefore, it must be removed from wastewaters before their discharged to ensure environmental safety [35]. Temperature, ionic strength, adsorbent dosage, pH, and the properties of the adsorbate all have an impact on adsorption process [32, 38]. Therefore, we optimized reaction conditions for optimum performance of the adsorbent. The performance of adsorbent was also checked in tap water and river water as well as in saline water, and it showed excellent adsorption efficiency for removal of CV dye. The main advantage of adsorbent is that it can be sued directly without any treatment which reduce cost of process and make it convenient to be used on large scale. Thus, Trifolium repens stem powder may be utilized on a large scale as a useful adsorbent to remove the harmful colors from the wastewater to save natural water bodies from pollution.
Materials and methods
Materials
All materials were employed without additional purification. In this work, the adsorbent used was Trifolium repens stem powder, while adsorbates were a cationic dyes CV, obtained from sigma (BDH laboratory ENGLAND). HCl (37%) and ethanol were obtained from MERCK GERMANY, NaOH was obtained from KOREA, and NaCl and kCl and MgCl2 were obtained from DAEJUNG.
Equipment
A digital orbital shaker was set at 200 rpm and operated at room temperature to ensure proper biosorbent and dye mixing (A product of PCSIR Pakistan). The UV–visible (Shimadzu Brand UV-3000, with a 1 cm path length) spectrophotometer was used to check absorbance.
Preparation of adsorbent
Fresh Trifolium repens plant stem was collected from local areas of Swabi. To get rid of water-soluble pollutants and impure particles, they were washed with tap water first, then with distilled water, then dried in sun for 72 h and then, crushed into powder. The sealed plastic bags were used to store the dried powder. The dried biomass was subjected to surface characterization and was used in adsorption investigations, without any treatment. Other part of this plant, i.e., leaves and seeds were also tested as adsorbent, but stem showed good adsorption capacity. Therefore, stem was chosen as adsorbent for the present study.
Preparation of dye solution
The dye solutions concentrations ranged from 10 to 100 mg/L. A 100 mg of dye was dissolved in 1 L distilled water to prepare a stock solution, and other solutions were prepared by using dilution formula. The pH of working solution was adjusted by adding 0.1 mol/L of an HCl and NaOH to the solution.
Determination of the adsorption efficiency of trifolium repens plant parts
The study was carried out to find out the adsorption capacity of Trifolium repens plant parts (stem, seeds and leaves) for CV dyes solution. For this purpose 0.2 g of stem, seed and leave were added to 50 mg/L (20 ml) solution of CV dye solution in a 100 ml beaker. Absorbance was noted by spectrophotometer with lambda max 585 nm and put on orbital shaker for 80 min. After this, a filter paper was used to filter all solutions and again their absorbance was noted. The final concentration of the dye solution was estimated using Beer–Lambert law [15, 29, 40]., which is
where A = absorbance of solution, Ɛ = constant, C = concentration of solution and l = path length of cuvette.
The Qe (mg/g) values, which show the dye adsorbed amount on the adsorbent’s surface, were calculated using formula given below [20].
where Ci (ppm) is the initial concentration of dye solution before adsorption, m (g) shows the adsorbent’s mass, V (L) is the solution’s volume, and Ce (ppm) is the equilibrium concentration of dye solution.
Similarly, % removal was calculated using the following formula [20].
Particle size analysis
The particle size of biosorbent (Trifolium repens) stem powder was determined using commercially available measuring instrument. All the adsorbents were ground between 3 particle size ranges: 1–1.25 mm large size, 0.4–0.5 mm medium size, and 0–0.1 mm small size. The dried form of biosorbent was shaken through series of sieves with various sizes of mesh (50, 60 and 100). The particle size was 0.297 mm (297 µm) which was sieved through 50 mesh sieves and was used in this study. The higher dye removal was owing to the large surface area of small particles sizes, as compared to small surface area of large particles sizes.
Adsorption experiments
CV dye solution was prepared by dissolving a weighted quantity of dye in distilled water to prepare stock solution. Then, the required concentration (10–100 ppm) was obtained through dilution formula. A varied quantity of adsorbent dosage was put into a 100 mL conical flask containing 30 mL (CV), and the pH was controlled with 0.1 M NaOH and 0.1 M HCl solution. The solution was shaken at 190 to 200 rpm on a shaker. The dye concentration was found out at characteristic wavelength (CV lambda max = 585 nm by double beam UV–visible 3000 (Shimadzu Brand UV-3000, with a 1 cm path length) spectrophotometer respective to the maximum absorbance by CV. Using predetermined experimental parameters, silica gel and charcoal were tested and compared with our study. The adsorbent performance was also checked in various natural water samples, such as river water and tap water and then, the impact of ionic strength on adsorption process was also checked. In all conditions, our adsorbent showed promising results.
Characterization of biosorbent
Scanning electron microscopy (SEM) was carried out using a (JEOL Electronics Company, Japan) scanning electron microscope (JSM-IT-100) that was employed to examine the surface structure of the biosorbent and Fourier transform infrared (FTIR) spectroscopy analysis with the help of FTIR spectrophotometer to determine the present chemical functional groups on the biosorbent (Trifolium repens). Thus, the FTIR spectra of Trifolium repens, before and after the biosorption of CV dye, were recorded with a FTIR spectrometer (IRAffinity-1S-Shimadzu). The absorbance data were collected for wavenumbers in the 500–4000 cm − 1 range.
Results and discussions
FTIR analysis
The FTIR spectrum analysis was used to determine the various functional groups at the biosorbent surface that cause the dye ion biosorption. The active groups and bonds exist on biomass or plant material are responsible for adsorption [33]. Trifolium repens mostly contains isoflavones, but certain species also produce a diverse spectrum of phenolic and polyphenolic chemicals, including saponins, flavonoids, clovamides, phenolic acids, and other compounds [2]. Peaks in the FTIR spectrum of biosorbent (Trifolium repens) were allocated to distinct groups and bonds. In Fig. 1, the FTIR spectra of biosorbent (Trifolium repens) before and after biosorption of CV are presented. The FTIR spectra of before sample of biosorbent (Trifolium repens) reveal multiple distinct and strong peaks in the range of 500 to 1700 cm−1 and 2500 to 3600 cm−1. A small absorbance peak was observed at 3610 cm−1 and 3538 cm−1 which indicate the existence of H–O group. From 3000 cm-1 to 3500 cm-1 (3440, 3410, 3332, 3190, 3162, 3125, 3050 cm-1), a broad and significant band stretch was found, suggesting the existence of free hydrogen linked O–H groups of phenols, alcohols, free H- bonded and carboxylic acid [37]. The stretching vibration of the C–H group of aldehyde, methyl, and methylene groups is shown by the peak at 2960, 2920 cm-1 [8, 9]. Before biosorption, broad bands in the 3000–3500 cm1 range showed O–H and N–H stretching vibrations, indicating the presence of alcohols, hydroxyl, and primary, secondary, and amide groups on the Trifolium repens surface. The stretching vibration of the C–O bond owing to non-ionic carboxyl groups (–COOH, –COOCH3) is noticed at 1780 cm−1 and may be ascribed to carboxylic acids or their esters [20]. The peaks in range of 1550 and 1580 cm−1 imply the C–C groups. The peak at 1485 cm1 implies the presence C=C [5]. A sharp peak in range of 1000 to 1350 cm−1(1130, 1210, 1260 cm−1 indicates the presence of C–O and C–C groups. The stretching vibration of C–OH of carboxylic acids and alcoholic groups may be responsible for the peaks at 1054 cm−1 [33]. Bands connected to aromatic, out of plane C=H and C–Cl bending with varying degree of substitution may be seen between 600 and 900 cm-1 [42].
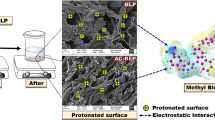
After adsorption of crystal violet dye onto biosorbent (Trifolium repens), sharp peaks arise at 3610 and 3525 cm−1, the distinctive OH band at 3538 cm−1 changes to 3525 cm−1, and the peak at 3332 shifts to 3335 cm−1. The bond moved from 1780, 1683 cm−1, and 1580 cm-1, to 1693 cm−1, 1612 cm−1, and 1534 cm−1, respectively. After CV dye biosorption, the peaks on the Trifolium repens surface changed, showing a chemical interaction between CV molecules and the OH group of alcohols, phenols, free H– bonded and carboxylic acids. Therefore, it can be presumed that the OH and COOH groups, as well as the C–H groups, are the primary binding sites for CV dye on adsorbent (Trifolium repens). The presence of carboxyl C–O and hydroxyl O–H groups in the FTIR spectra of biosorbent (Trifolium repens) is clearly demonstrated. The electrostatic interaction between these groups and the cationic dye molecules (CV) shows CV dye sorption on Trifolium repens biomass.
The carboxylic groups are deprotonated at pH 2, allowing negatively charged carboxylate ligands (–COO) to attach to positively charged CV molecules. This ion exchange process between the cationic dye molecule and the negatively charged groups in biosorbent (Trifolium repens) cell wall is responsible for CV dye sorption by adsorbent (Trifolium repens) [5].
Characterization of biosorbent surfaces by scanning electron microscopy
Before and after adsorption, the surface structure of the biosorbent (Trifolium repens) was completely different as indicated by SEM characterization. Before adsorption, a rough surface morphology with a particle size of 50 μm was seen, however, when the dye was adsorbed on the biosorbent (Trifolium repens), a layer on the surface of the adsorbent and a smoother morphology are seen in Fig. 2. These image showed that the surfaces of the loaded (dye) and unloaded (biosorbent) had different morphologies. The unloaded biosorbent possesses a morphologically rough surface and some porous cavities. After loading of CV dye, the biosorbent surface was altered and the small particles accumulate which stick to each other to create a multilayer on the surface of the biomass.
Comparison of adsorption performance of trifolium repens tree different parts CV dye removal
Different parts of Trifolium repens plant, i.e., seeds, leaves and stem as adsorbents with their percent removal are given in Fig. 3. After absorbance, the results clearly represent that adsorption capacity of Trifolium repens stem was greater than leaves and seeds. The stem of white clover shows 83.48% removal of (CV) dye. Therefore, Trifolium repens stem was used as an adsorbent for further study.
Influence of initial dye concentration on adsorption
CV dye solutions of several concentrations (10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 mg/L) were prepared to check the influence of initial dye concentration on adsorption. A 100 mg adsorbent dose was added to different 30 ml of different dye solutions and put on shaker for 80 min. After shaking, a filter paper was used to filter solutions and their absorbance values were noted. It can be observed in Fig. 3 that the percent removal was increased as the concentration was increased from 10 to 70 mg/L. After 70 mg/L, no further increase in percent removal was seen for an additional increase in concentration. This might be because all biosorbents have a finite number of binding sites, which get saturated when the dye concentration is high and the CV concentration reaches equilibrium between the liquid and solid phases [6].
As dye concentration was raised from 10 to 70 ppm, the value of percent removal increased from 79.977 to 82.268 and the Qe value increased from 2.399 to 17.276 as seen in Fig. 4. After 70 ppm, no further increase in percent removal was observed. This initial rise in percent removal with increase in dye concentration is because of the fact that at low dye concentration, greater number of active sites on the surface of adsorbent are available for adsorption of dye and all sorbents have a finite number of binding sites that saturate at a particular concentration [7, 21]. No further increase in percent adsorption at higher concentration was due to saturation of surface-active sites, and equilibrium was established between adsorbed and dye that was present in solution at equilibrium. So, 70 ppm was the optimum concentration for this research work. It means that 100 mg of Trifolium repens (adsorbent) has the ability to efficiently adsorb 70 ppm of CV dye (adsorbate) from 30 ml solution.
Influence of pH on crystal violet dye adsorption
The adsorption of dyes at the surface of adsorbent is also influenced by solution pH, because change in pH affects the surface charge on the adsorbent. Batch adsorption experiment was brought out by changing pH of solution from 1 to 10 by keeping other experimental conditions constant (adsorbent dose 0.1 g, 30 ml solution of CV dye at concentrations 70 mg/L and contact time 80 min). The pH was controlled by using 0.1 M HCl and 0.1 M NaOH solutions. The variations in the removal of CV dye with pH are given in Fig. 5. The results show that the percentage removal of CV dye is highest at pH 2 and then, decreases slightly with increasing pH, after which it remains almost constant. This might be due to change in adsorbate structure, and the functional groups present at the surface of adsorbent may change with change in pH and can be protonated or deprotonated to generate different surface charges at different pH in the solutions, leading to an electrostatic attraction/repulsion between the charges adsorbents and adsorbates [3, 7].
Influence of adsorbent dose for removal of CV dye
The dosage of the adsorbent is another key factor that influences the adsorption process. The adsorption capacity of adsorbent dosage was determined by adding different quantities of adsorbent dose ranging from 0.1 to 1 g, keeping fixed other experimental conditions, i.e., pH (2), contact time (80 min), and initial dye concentration (70 mg/L). The percent adsorption of CV at different adsorbent doses is given in Fig. 6, which indicates that the percent adsorption of CV onto adsorbents rises with increasing adsorbent dose and then, becomes constant and no further increase occurs with an increase in the adsorbent dose. The rise in the percent adsorption of the dye with raising the adsorbent dose can be attributed to the larger adsorbent surface-active sites availability to the fixed number of dye molecules. The adsorption capacity (mg/g) of adsorbents lowers from 16.287 to 1.921 mg/g with raising the adsorbent dose from 0.1 to 1 g. The adsorption capacity of the adsorbent lowers with raising the adsorbent quantity owing to the accumulation of the adsorbent particles at higher adsorbent doses, because of which some active surface sites of the adsorbent remain unavailable for the dye molecules’ absorption [5].
Impact of contact time on the process of adsorption
The adsorption study was performed at different time intervals ranging from 20 to 160 min to determine the equilibrium contact time for the removal of CV dye from the aqueous solutions. Other experimental conditions like pH of 2, initial dye concentration of 70 mg/L (30 ml), adsorbent dose of 1.0 g were kept constant. The experimental results are given in Fig. 7. As seen the figure, the CV adsorption onto adsorbent rises with increasing contact time up to 140 min and then, almost remains constant after the 140 min. Hence, the equilibrium contact time for the CV adsorption onto adsorbent was 140 min and further experimental study was performed at 140 min.
Influence of salt concentration on adsorption
Some salts are employed in the dyeing and coloring of fabrics. The salt content in dye-containing effluent is usually greater. As a result, it is crucial to investigate the impact of ionic strength in dye adsorption on adsorbent. For this purpose, the optimum conditions (70 ppm of CV dye (30 ml) with 1 g adsorbent, pH 2) were used to prepared dye solution in distilled and tap water to know the effect of salts on adsorption process. The impact of salt concentration on the dyes’ biosorption process was assessed by the addition of sodium chloride, potassium chloride and manganese chloride salts to the CV dye solution. Their absorbance values were determined by using of UV/Vis spectroscopy. The concentration of salts used 0.05 g/30 ml solution. The presence of Na + , K + , Mg2+ and Cl−1 ions resulted in a slight decrease in dye removal in both samples of water as seen in Fig. 8. The expansion of the electrical diffused double layer in aqueous solution inhibits the adsorbent and basic dye ions from contacting each other, because of competitive effect between dye ions and cations from the salt sites available for the adsorption process, and a reduction in electrostatic attraction and adsorption efficiency [3, 17]. From the results, it can be seen that the percent removal is still very high for the adsorbent under study, and thus, it can be used in saline water and in real industrial water for efficient removal of CV dye.
Adsorption isotherms
Isotherms offer the most essential knowledge for understanding the biosorption process which play a key role in the design and analysis of a biosorption process. The isotherm models' equation parameters and fundamental thermodynamic concept provide some information regarding the biosorption mechanism and the surface characteristics as well as affinity of the biosorbent [8, 9]. For the determination of equilibrium data, the two isotherm that are Langmuir [31] and Freundlich [34] isotherm equations were applied. Adsorption isotherms elaborate the relationship between adsorbent and adsorbate materials. Freundlich isotherm has been employed to examine the experimental data. Freundlich isotherm also presumes multilayer sorption on heterogeneous adsorbent surface. The linearized version of the equation can be given as:
where Kf (l/g) is the Freundlich constant, showing the capacity of adsorption and n is the Freundlich exponent that shows the intensity of adsorption. The exponent, 1/n, indicates the adsorption favorability. An n > 1 implies favorable adsorption conditions [3]. The values of log(qe) and log(Ce) of the linear Freundlich isotherm of CV dye plot are shown in Fig. 9a.
Langmuir isotherm model represents adsorption that occurs at the specific homogeneous (monolayer) sites on the adsorbent. The linearized form of Langmuir isotherm is given:
where, qe (mg/g) is the quantity of dye adsorbed per unit adsorbent’s mass at equilibrium time, Ce (mg/l) is the dye concentration at equilibrium in the solution, qmax (l/mg) are the Langmuir constants associated with the maximum monolayer adsorption capacity. These numbers are estimated from the slope and intercept from the plots of 1/qe versus 1/ce (Fig. 9b).
The results indicate that CV dye was favorably adsorbed by absorbent (Trifolium repens) with high correlation coefficient values (R2 = 0.998). It indicates that the Langmuir isotherm was best fitted for the CV dye’s adsorption on Trifolium repens. Table 1 represents the Freundlich and Langmuir adsorption isotherm values.
It is revealed that the equilibrium data best fit in Langmuir isotherm which suggested the monolayer sorption of crystal violet dye on white clover (adsorbent). A Freundlich constant n (> 0) and larger value of qmax indicate stronger interaction between CV dye and Trifolium repens stem powder. Trifolium repens stem powder, due to its huge availability from the local supply and high biosorption intensity for CV, is a valuable low-cost biosorbent for the economical treatment of CV contaminated water.
Kinetic study
Developing industrial scale separation techniques requires an understanding of the kinetics biosorption processes. To examine the kinetics of the biosorption process, the data from the contact time dependent study were employed. The pseudo-first-order (vi) and pseudo-second-order kinetic models (vii) have been applied to find the rate constants and explain the underlying biosorption mechanism [8, 9]. The investigation of adsorption kinetics explains the solute uptake rate. The linear form of pseudo-first-order equation [25, 39] is given as follows:
where qt and qe are the adsorption amount at time t and at equilibrium, respectively, and (K1) is the rate constant of the pseudo-first-order adsorption. A plot of ln (qe − qt) Vs t (Fig. 10 (a)) needs to be linear, and adsorption capacity at equilibrium (qe) and rate constant (K1) can be found out from the intercept and the slope of the plot, respectively. The R2 values indicate that the first-order equation could not properly fit the full range of the adsorption process. The linear pseudo-second-order equation [18] is given as follows:
where (K2) is the pseudo-second-order rate constant. The slope of the t/qt versus t plot helps to measure the value of qe, and the intercept can be employed for the calculation of (K2). The t/qt versus t plot (see Fig. 10b results in a very straight line. The correlation coefficient (R2) for the model is 0.992, implying a better fit to our data. The parameters calculated from pseudo-first-order and pseudo-second-order equation are given in Table 2.
Determination of adsorption performance of trifolium repens stem powder in water collected from different sources
The performance of the adsorbent was also checked in water collected from various sources, including tap water, river water, and distilled water to test the adsorbent for its practical use in real water. For this purpose, a 30 ml of 70 ppm CV dye solution was prepared under optimized conditions (1 g dose, pH 2, 140 min), using different water samples to know the effectiveness of adsorbent. After shaking, the solution was filtered with filter paper and their absorbance was noted by using UV/Vis spectroscopy. The results show that the percent removal of dye in different water samples was 78.005% river water, 86.02% distal water and 86.69% tap water. This showed that the adsorbent prepared is effective to treat wastewater practically and the adsorbent showed excellent performance in all water samples (Fig. 11). Thus, it can be concluded that this adsorbent can be utilized to effectively remove contaminated colors from industrial wastewater.
Comparison of adsorption efficiency of trifolium repens stem powder, animal charcoal and silica gel for CV dye
The removal efficiency of adsorbent was also compared with other commercially available adsorbents that are animal charcoal and silica gel. For this purpose, a 70 ppm of CV dye solution was prepared under optimized conditions and their pH was maintained at 2. Then, 30 ml volume of dye solution was taken in three 100 ml beakers using a pipette. Their absorbance values were noted by UV/Vis spectrophotometer. A 1 g of each of animal charcoal, silica gel, and Trifolium repens plant stem powder was added to these solutions and placed on a digital orbital shaker for optimum time. After this, all solutions were filtered with filter paper and again their absorbance values were noted. From Fig. 12, it can be seen that Trifolium repens plant stem powder has a greater affinity for the adsorption of CV dye like silica gel and animal charcoal. The adsorbent (Trifolium repens) removes 93.12% of crystal violet dye, while animal charcoal and silica gel remove 92.35% and 95.002% of crystal violet dye, respectively.
The performance of our biosorbent was also compared with the biosorbents used in the reported literature. As it can be seen in Table 3, our biosorbent show a comparable performance when compared with previously reported studies. Additionally, the adsorbent (Trifolium repens) is cost-effective, widely available and requires no treatment, making it a more suitable adsorbent.
Conclusion and recommendations
This study indicates that Trifolium repens stem powder has adsorptive characteristics and capabilities and thus, can be employed as an adsorbent to remove the dye from aqueous solutions and industrial effluent. The batch adsorption investigations were done by optimizing various parameters such as adsorbent dosage, contact time, pH, and starting dye concentration for CV dye removal onto absorbent (Trifolium repens). As the absorbent surface was occupied by hydroxyl groups, it had high electrostatic adsorption capabilities for various cationic dyes. It has been shown that the pseudo-second-order kinetic model better expresses the adsorption kinetic of CV with R2 of 1. The adsorption was single layer adsorption as per the Langmuir model. The findings of this study are important for environmental applications and as a Trifolium repens plant step can be employed as an economically viable adsorbent for the removal of hazardous dyes from wastewater without any treatment. The adsorbent also showed excellent results in different saline water, and river and tap water; thus, it can be directly used at commercial level without any treatment for removal of contaminating dyes from industrial wastewater.
Data availability
The data sets used in this study are available on reasonable request.
References
R. Ahmad, Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J. Hazard. Mater. 171, 767–773 (2009)
S. Ahmad, A. Zeb. Phytochemical profile and pharmacological properties of Trifolium repens. J. Basic Clin. Physiol. Pharmacol. 32(1) (2021)
M. Alshabanat, G. Alsenani, R. Almufarij,. Removal of crystal violet dye from aqueous solutions onto date palm fiber by adsorption technique. J. Chem. (2013)
A. Bano, F. Khitab, R. Khattak, S. Rasool, H. Gul, Enhanced photocatalytic activity and charge carrier separation of a nickel impregnated zinc oxide catalyst for the visible photodegradation of remazol brilliant violet-5R and methyl orange. Anal. Lett. 56, 1–13 (2022)
O.S. Bello, M.A. Ahmad, Coconut (Cocos nucifera) shell based activated carbon for the removal of malachite green dye from aqueous solutions. Sep. Sci. Technol. 47(6), 903–912 (2012)
S. Chakraborty, S. Chowdhury, P.D. Saha, Insight into biosorption equilibrium, kinetics and thermodynamics of crystal violet onto Ananas comosus (pineapple) leaf powder. Appl Water Sci 2(2), 135–141 (2012)
G.K. Cheruiyot, W.C. Wanyonyi, J.J. Kiplimo, E.N. Maina, Adsorption of toxic crystal violet dye using coffee husks: equilibrium, kinetics and thermodynamics study. Sci. Afr. 5, e00116 (2019)
S. Chowdhury, R. Mishra, P. Saha, P. Kushwaha, Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265(1–3), 159–168 (2011)
S. Chowdhury, R. Misra, P. Kushwaha, P. Das, Optimum sorption isotherm by linear and nonlinear methods for safranin onto alkali-treated rice husk. Bioremediat. J. 15(2), 77–89 (2011)
P. Dutta, M. Rabbi, M. Abu Sufian, S. Mahjebin, Effects of textile dyeing effluent on the environment and its treatment: a review. Eng. Appl. Sci. Lett. 5(1), 1–1 (2022)
S. Dutta, J. Bhattacharjee, A comparative study between physicochemical and biological methods for effective removal of textile dye from wastewater (Development in Wastewater Treatment Research and Processes (pp. 1–21). Elsevier (2022)
G.A. El Naeem, A. Abd-Elhamid, O.O. Farahat, A.A. El-Bardan, H.M. Soliman, A. Nayl, Adsorption of crystal violet and methylene blue dyes using a cellulose-based adsorbent from sugercane bagasse: characterization, kinetic and isotherm studies. J. Market. Res. 19, 3241–3254 (2022)
K. Elsherif, A. El-Dali, A. Alkarewi, A. Ewlad-Ahmed, A. Treban, Adsorption of crystal violet dye onto olive leaves powder: equilibrium and kinetic studies. Chem. Int. 7(2), 79–89 (2021)
S. Gul, A. Gul, H. Gul, R. Khattak, M. Ismail, S.U. Khan, M.S. Khan, H.A. Aouissi, A. Krauklis, Removal of brilliant green dye from water using Ficus benghalensis tree leaves as an efficient biosorbent. Materials 16(2), 521 (2023)
S. Gul, H. Gul, M. Gul, R. Khattak, G. Rukh, M.S. Khan, H.A. Aouissi, Enhanced adsorption of rhodamine b on biomass of cypress/false cypress (Chamaecyparis lawsoniana) fruit: optimization and kinetic study. Water 14(19), 2987 (2022)
S. Gul, M. Kanwal, R.A. Qazi, H. Gul, R. Khattak, M.S. Khan, F. Khitab, A.E. Krauklis, Efficient removal of methyl red dye by using bark of hopbush. Water 14(18), 2831 (2022)
N. Gupta, A.K. Kushwaha, M. Chattopadhyaya, Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arab. J. Chem. 9, S707–S716 (2016)
Y.-S. Ho, G. McKay, Sorption of dye from aqueous solution by peat. Chem. Eng. J. 70(2), 115–124 (1998)
P.L. Homagai, R. Poudel, S. Poudel, A. Bhattarai, Adsorption and removal of crystal violet dye from aqueous solution by modified rice husk. Heliyon 8(4), e09261 (2022)
N. A. Hussain, A. Taifi, O.K. A. Alkadir, N. H. Obaid, Z. M. Abboud, A. M., Aljeboree, A. L. Al Bayaa, S. A. Abed, A. F. Alkaim, Role of pomegranate peels as a activated carbon for removal of pollutants, in IOP Conference Series: Earth and Environmental Science.
M. Ishaq, F. Javed, I. Amad, H. Ullah, F. Hadi, S. Sultan, Adsorption of crystal violet dye from aqueous solutions onto low-cost untreated and NaOH treated almond shell. Iran. J. Chem. Chem. Eng. 35(2), 97–106 (2016)
M. Karimi-Nazarabad, H. Azizi-Toupkanloo, Functionalization of beet waste by cross-linking to attach amine groups for efficient sorption of reactive black 5 anionic dye. J. Iran. Chem. Soc., pp. 1–11 (2021)
M. Karimi-Nazarabad, E.K. Goharshadi, R. Mehrkhah, M. Davardoostmanesh, Highly efficient clean water production: reduced graphene oxide/graphitic carbon nitride/wood. Sep. Purif. Technol. 279, 119788 (2021)
P.S. Kumar, G.J. Joshiba, C.C. Femina, P. Varshini, S. Priyadharshini, M. Karthick, R. Jothirani, A critical review on recent developments in the low-cost adsorption of dyes from wastewater. Desalin. Water Treat 172, 395–416 (2019)
S.K. Lagergren, About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 24, 1–39 (1898)
H.M. Mashhoor, M. Eftekhari, N. Rezazadeh, M.K. Nazarabad, Graphene oxide–tungsten oxide (GO–WO3) adsorbent for the removal of copper ion. Nanotechnol. Environ. Eng. 8(1), 75–86 (2023)
R. Mehrkhah, E.K. Goharshadi, M.M. Ghafurian, M. Mohammadi, O. Mahian, Clean water production by non-noble metal/reduced graphene oxide nanocomposite coated on wood: scalable interfacial solar steam generation and heavy metal sorption. Sol. Energy 224, 440–454 (2021)
R. Mehrkhah, E.K. Goharshadi, M. Mohammadi, Highly efficient solar desalination and wastewater treatment by economical wood-based double-layer photoabsorbers. J. Ind. Eng. Chem. 101, 334–347 (2021)
A. O. Odiongenyi influence of sol gel conversion on the adsorption capacity of crab shell for the removal of crystal violet from aqueous solution. Commun. Phys. Sci., 8(1) (2022)
G. O. Ogunlusi, O. D. Amos, O. F. Olatunji, A. A. Adenuga, Equilibrium, kinetic, and thermodynamic studies of the adsorption of anionic and cationic dyes from aqueous solution using agricultural waste biochar. J. Iran. Chem. Soc., pp. 1–14 (2022)
C.M. Oloo, J.M. Onyari, W.C. Wanyonyi, J.N. Wabomba, V.M. Muinde, Adsorptive removal of hazardous crystal violet dye form aqueous solution using Rhizophora mucronata stem-barks: equilibrium and kinetics studies. Environ. Chem. Ecotoxicol. 2, 64–72 (2020)
M.S.U. Rehman, M. Munir, M. Ashfaq, N. Rashid, M.F. Nazar, M. Danish, J.-I. Han, Adsorption of brilliant green dye from aqueous solution onto red clay. Chem. Eng. J. 228, 54–62 (2013)
A. Saeed, M. Sharif, M. Iqbal, Application potential of grapefruit peel as dye sorbent: kinetics, equilibrium and mechanism of crystal violet adsorption. J. Hazard. Mater. 179(1–3), 564–572 (2010)
P.D. Saha, S. Chakraborty, S. Chowdhury, Batch and continuous (fixed-bed column) biosorption of crystal violet by Artocarpus heterophyllus (jackfruit) leaf powder. Colloids Surf., B 92, 262–270 (2012)
S. Sultana, K. Islam, M.A. Hasan, H.J. Khan, M.A.R. Khan, A. Deb, M. Al Raihan, M.W. Rahman, Adsorption of crystal violet dye by coconut husk powder: isotherm, kinetics and thermodynamics perspectives. Environ. Nanotechnol. Monit. Manage. 17, 100651 (2022)
R. Sulthana, S. N. Taqui, U. T. Syed, T. Khan, S. D. A. Khadar, I. Mokashi, K. Shahapurkar, M. Kalam, H. Murthy, A. A. Syed, Adsorption of crystal violet dye from aqueous solution using industrial pepper seed spent: equilibrium, thermodynamic, and kinetic studies. Adsorp. Sci. Technol. 2022.
B.K. Suyamboo, R. Srikrishnaperumal, Biosorption of crystal violet onto cyperus rotundus in batch system: kinetic and equilibrium modeling. Desalin. Water Treat. 52(19–21), 3535–3546 (2014)
S.J. Ukkund, P. Puthiyillam, H.M. Alshehri, M. Goodarzi, S.N. Taqui, A.E. Anqi, M.R. Safaei, M.A. Ali, U.T. Syed, R.A. Mir, Adsorption method for the remediation of brilliant green dye using halloysite nanotube: isotherm, kinetic and modeling studies. Appl. Sci. 11(17), 8088 (2021)
T. Ullah, H. Gul, F. Khitab, R. Khattak, Y. Ali, S. Rasool, M.S. Khan, I. Zekker, Adsorption of Remazol brilliant violet-5R from aqueous solution using sugarcane bagasse as biosorbent: kinetic and thermodynamic studies. Water 14(19), 3014 (2022)
Urooj, M., & Fatima, R. ADSORPTION BEHAVIOR OF A MODEL AZO DYE ON SURFACTANT MODIFIED ALUMINA BASED ADSORBENT.
Y.-G. Zhao, Y. Zhu, Y. Zhang, M.-L. Ye, Ultrasound-assisted synthesis of tetraethylenepentamine-modified graphene oxide/dispersive Fe 3 O 4 composites with enhanced adsorption capacity for allergenic disperse dyes. J. Iran. Chem. Soc. 18, 1113–1125 (2021)
W. Zou, S. Gao, X. Zou, H. Bai, Adsorption of neutral red and malachite green onto grapefruit peel in single and binary systems. Water Environ. Res. 85(5), 466–477 (2013)
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose. All authors gave the final approval for publication and agreed to be held accountable for the work performed herein.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gul, S., Afsar, S., Gul, H. et al. Removal of crystal violet dye from wastewater using low-cost biosorbent Trifolium repens stem powder. J IRAN CHEM SOC 20, 2781–2792 (2023). https://doi.org/10.1007/s13738-023-02875-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02875-x