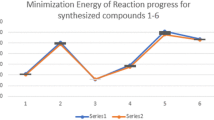
Abstract
The furanone 1 was allowed to react with different nitrogen nucleophiles such as hydrazine hydrate, hydroxyl amine, anisidine, anthranilic acid, glycine, ethylglycinate and isatin and some carbon nicleophiles under Michael reaction conditions assisted by microwave irradiation. They afforded new synthesized some important heterocyclic compounds 2–16. The structures of the synthesized new compounds were characterized by spectral data and elemental analysis. DFT calculation is serving to clarify not only the active electrophilic sites attacked by suitable nucleophiles in the organic reaction but also the bioactivity of the larvicidal compounds. The larvicidal activities of the synthesized compounds were tested against Plutella xylostella and Helicoverpa armigera larvae in vitro. The results revealed that the synthesized compounds 4, 13 and 14 showed good insecticidal activity against Plutella xylostella larvae with 100%, 87.2% and 91.4% activities, respectively, while 13 and 14 displayed 92% and %, 94.8 activities, respectively, against Helicoverpa armigera larvae. Therefore, they exhibited good larvicidal activity. The executed molecular docking simulations evinced the selective binding of the novel synthesized compounds toward acetylcholinesterase (AchE) of Drosophila Melanogaster, which signifies their role as a prominent larvicides. Compound 4, 13 and 14 showed clear docking affinity and to the assumed binding sites in terms of both calculated binding energies and the types of interactions, which provided a solid rationalization for the conducted experimental results.











Similar content being viewed by others
References
K. Subbaramaiah, A.J. Dannenberg, Trends Pharmacol. Sci. 24, 96–102 (2003)
M.A. Iniguez, A. Rodriguez, O.V. Volpert, M. Fresno, J.M. Redondo, Trends Mol. Med. 9, 73–78 (2003)
E. Nvarro, S.J. Alonso, J.E. Trujillo, C. Jorge, C. Perez, J. Nat. Prod. 64, 134–135 (2001)
E. Gondela, K.Z. Walczak, Eur. J. Med. Chem. 45, 3993–3997 (2010)
R. Surmont, G. Verniest, N. De Kimpe, J. Org. Chem. 75, 5750–5753 (2010)
Y.Q. Mo, Z.Y. Wang, W.J. Mei, J.H. Fu, Y.H. Tan, S.H. Luo, Monatsh. Chem. 143, 443–453 (2012)
S.S. Maigali, M. El-Hussieny, F.M.J. Soliman, Het. Chem. 52, 15–23 (2015)
M. Aarts, R. Sharpe, I. Garcia-Murillas, H. Gevensleben, M.S. Hurd, S.D. Shumway, N.C. Turner, Cancer Discov. 2(6), 524–539 (2012)
A.E. Elkholy, S.A. Rizk, A.M. Rashad, J. Mol. Struct. 1175, 788–796 (2019). https://doi.org/10.1016/j.molstruc.2018.08.045
M. El-Hashash, S.A. Rizk, M.M. Aburzeza, Egypt. J. Chem. 54(3), 299–312 (2011)
J.Y. Cho, K.U.B.E. Sook Yoo, K. Yoshikawa, M. Park, H. J. Nat. Prod. 63, 1205–1209 (2000)
M.-X. Wei, L. Feng, X.-Q. Li, X.-Z. Zhou, Z.-H. Shao, Eur. J. Med. Chem. 44, 3340–3344 (2009)
K.R. Prasad, V. Gandi, R. Tetrahedron Asymmetry 21, 2848–2852 (2010)
M.L.C. Borsato, C.F.F. Grael, G.E.P. Souza, N.P. Lopes, Phytochemistry 55, 809–813 (2000)
M. El-Hashash, S.A. Rizk, A. El-Badawy, J. Het. Chem. 55, 2090–2098 (2018). https://doi.org/10.1002/jhet.3250
M.M. Alam, A. Husain, S.M. Hasan, S. Khanna, M. Shaquiquzzaman, J. Enzyme Inhib. Med. Chem. 25(3), 323–330 (2010)
R. Sasidharana, M.S. Leelabaiamma, R. Mohanan, S.P. Joseb, B. Mathew, S. Sukumar, Immunopharmacol. Immunotoxicol. 41(6), 1–9 (2019)
E. Lattmann, S. Dunn, S. Niamsanit, N. Sattayasai, Bioorg. Med. Chem. Lett. 15, 919–921 (2005)
S.A. Rizk, S.S. Abdelwahab, H.A.J. Sallam, Het. Chem. 55, 1604–1614 (2018). https://doi.org/10.1002/jhet.3195
M.D. Guerrero, M. Aquino, I. Bruno, M.C. Terencio, M. Paya, R. Riccio, L. Gomez-Paloma, J. Med. Chem. 50, 2176–2184 (2007)
H.V.E. Juan, J.R. Saad, O.S. Giordano, C. Garcia, T. Martin, V.S. Martin, M.E. Sosa, C.E. Tonn, J. Nat. Prod. 71, 190–194 (2008)
T. Ukita, M. Sugahara, Y. Terakawa, T. Kuroda, K. Wada, A. Nakata, Y. Ohmachi, H. Kikkawa, K. Ikezawa, K. Naito, J. Med. Chem. 42, 1088–1099 (1999)
A. Shojaii, M. Motaghinejad, S. Norouzi, M. Motevalian, Iran J. Pharm Res. 14(1), 263–269 (2015)
E. Lattmann, J. Sattayasai, C.H. Schwalbe, Y. Boonprakob, S. Dunn, F. Fajana, P. Lattmann, Arch. Pharm. Chem. Life Sci. 349, 456–465 (2016)
X. Niu, R. Qin, Y. Zhao, L. Han, J. Lu, C. Lv, Biomed. Chromatog. 33, 4624 (2019). https://doi.org/10.1002/bmc.4624
M. El-Hashash, S.A. Rizk, J. Het. Chem. 53, 1236–1240 (2016). https://doi.org/10.1002/jhet.2375
T. Ukita, Y. Nakamura, A. Kubo, Y. Yamamoto, M. Takahashi, J. Kotera, T. Ikeo, J. Med. Chem. 42, 1293–1305 (1999)
A.F. Ademar, S. Albuquerque, L.A. Silva, M.N. Eberlin, M. Tomazela, K. Bastos, J. Nat. Prod. 67, 42–45 (2004)
N. Metropolis, A.W. Rosenbluth, M.N. Rosenbluth, A.H. Teller, E. Teller, J. Chem. Phys. 21, 1087–1092 (1953)
Y.K. Ramtohup, M.N.G. James, J.C. Vederas, J. Org. Chem. 67, 3169–3174 (2002)
Z. Xiang, L. Ho, J. Valdellon, D. Borchelt, K. Kelley, L. Spielman, P.S. Aisen, G.M. Pasinetti, Neurobiol. Aging 23, 327–334 (2002)
Z.H. Feng, T.G. Wang, D.D. Li, P. Fung, B.C. Wilson, B. Liu, S.F. Ali, R. Langenbach, J.S. Hong, Neurosci. Lett. 329, 354–358 (2002)
C. Michaux, X. de Leval, F. Julémont, J.M. Dogné, B. Pirotte, F. Durant, Eur. J. Med. Chem. 41, 1446–1455 (2006)
B.M. Trost, M.L. Crawley, J. Am. Chem. Soc. 124, 9328–9329 (2002)
D. Semenok, J. Medvedev, L.-P. Giassafaki, I. Lavdas, I.S. Vizirianakis, P. Eleftheriou, A. Geronikaki, Molecules 24(9), 1751 (2019)
R. Bhatiya, A. Vaidya, S.K. Kashaw, A.K. Jain, R.K.J. Agrawal, Saudi Chem. Soc. 18, 977–984 (2014)
B. Doroh, G.A. Sulikowski, Org. Lett. 8, 903–906 (2006)
V. Hickmann, A. Kondoh, B. Gabor, M. Alcarazo, A. Furstner, J. Am. Chem. Soc. 133, 13471–13480 (2011)
A. Benz, F. Cardona, A. Goti, Synlett 2, 231–234 (2011). https://doi.org/10.1055/s-0030-1259293
S.A. Rizk, G. Elsayed, M.A.J. El-Hashash, Iran. Chem. Soc. 15, 2093–2105 (2018)
S.A. Rizk, M. El-Hashash, A. El-Badawy, J. Het. Chem. 54, 2003–2011 (2017). https://doi.org/10.1002/jhet.2797
M.A. El-Hashash, K.M. Darwish, S.A. Rizk, F.A. El-Bassiouny, Pharmaceuticals 4(7), 1032–1051 (2011). https://doi.org/10.3390/ph4071032
A.F.M. Fahmy, S.A. Rizk, M.M. Hemdan, A.A. El-Sayed, A.I.J. Hassaballah, Het. Chem. 55, 2545–2555 (2018)
P.O. Patil, S.B. Bari, S.D. Firke, P.K. Deshmukh, S.T. Donda, D.A. Patil, Bioorg. Med. Chem. 21(9), 2434–2450 (2013)
H.C. Huang, F. Yu, Z.C. Jin, W.J. Li, W.B. Wu, X.M. Liang, J.X. Ye, Chem. Commun. (2010). https://doi.org/10.1039/C0CC01054E
A. Gassama, C. Ernenwein, N. Hoffmann, Green Chem. 12, 859–865 (2010)
H. Ube, N. Shimada, M. Terada, Angew. Chem. Int. Ed. 49, 1858–1861 (2010)
X.J. Wang, H.W. Xu, L.L. Guo, E. Zhang, G.W. Chen, X. Guo, H.M. Liu, Heterocycles 83, 1005–1012 (2011)
T. Gendrineau, O. Chuzel, H. Eijsberg, J.-P. Genet, S. Darses, Angew. Chem. Int. Ed. 47, 7669–7672 (2008)
Y. Du, L.W. Xu, Y. Shimizu, K. Oisaki, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 130, 16146–16147 (2008)
B.M. Trost, J. Hitce, J. Am. Chem. Soc. 131, 4572–4573 (2009)
J. Luo, H.F. Wang, X. Han, L.W. Xu, J. Kwiatkowski, K.W. Huang, Y.X. Lu, Angew. Chem. Int. Ed. 50, 1861–1864 (2011). https://doi.org/10.1002/anie.201006316
L. Zhou, L.L. Lin, J. Ji, M.S. Xie, X.H. Liu, X.M. Feng, Org. Lett. 13, 3056–3059 (2011)
S. Pares, P. de March, J. Font, R. Alibes, M. Figueredo, Eur. J. Org. Chem. (2011). https://doi.org/10.1002/ejoc.201100067
J.J. Yang, H.C. Huang, Z.C. Jin, W.B. Wu, J.X. Ye, Synthesis (2011). https://doi.org/10.1055/s-0030-1260052
E. Gondela, K.Z. Walczak, Molecules 16, 1011–1020 (2011)
M.A. El-Hashash, S.A. Rizk, S.R. Atta-Alla, Molecules 20(12), 22069–22083 (2015). https://doi.org/10.3390/molecules201219827
M.A. El-Hashash, S.A. Rizk, F.A. El-Bassiouny, D.B. Guirguis, S.M. Khairy, Egypt J. Chem. 60, 407 (2017). https://doi.org/10.21608/EJCHEM.2017.915.1043
X.S. Shao, H. Fu, X.L. Xu, Z.W. Liu, Z. Li, X.H. Qian, J. Agric. Food Chem. 58, 2696–2702 (2010)
K.G. Gorman, G. Devine, J. Bennison, P. Coussons, N. Punchard, I. Denholm, Pest Manag. Sci. 63, 555–558 (2007)
D.M. Sanchez, R.M. Hollingworth, E.J. Grafius, D.D. Moyer, Pest Manag. Sci. 62, 30–37 (2006)
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rizk, S.A., Atta-Allah, S.R. Microwave-assisted regioselective reaction of furanone derivative supported by DFT stimulation and molecular docking to afford controlling insecticidal agents. J IRAN CHEM SOC 18, 2407–2423 (2021). https://doi.org/10.1007/s13738-021-02202-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02202-2