Abstract
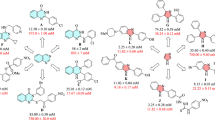
Functionalized quinazolinone derivatives 1–30 were synthesized by two-step reaction. First, anthranilic acid was treated with substituted phenyl isothiocyanate to synthesize 3-aryl-2-thioxo-2,3-dihydroquinazolinone derivatives 1–8 which in turn reacted with different bromoacetophenone derivatives to obtain fully functionalized quinazolinone derivatives 9–30. Both reactions were catalyzed by triethylamine. All the products were characterized by EI-, HREI-MS, 1H-, and 13CNMR spectroscopic techniques. All compounds were subjected to their in vitro α-glucosidase inhibitory activity. Results showed that except compound 1–3, 5, 7, and 22, all compounds were found potent and showed many folds increased α-glucosidase enzyme inhibition as compared to standard acarbose (IC50 = 750.0 ± 10.0 µM). Compound 13 (IC50 = 85.0 ± 0.5 µM) was recognized as the most potent analog of the whole series, with ninefold enhanced inhibitory potential than the standard acarbose. Compounds 1–9, 11, 12, 22, and 26 were structurally known compounds, while remaining all are new. Kinetic study on compound 13 showed that the compound is following a competitive-type inhibition mechanism. Furthermore, in silico studies have also been performed to better rationalize the interactions between synthetic compound and active site of the enzyme.
Graphic abstract











Similar content being viewed by others
References
S. Ibrahim, A. Al-Ahdal, A. Khedr, G. Mohamed, Rev. Bras. Farmacogn. 27, 170 (2017)
J.C. Burani, L. Rao, Good Carbs, Bad Carbs: An Indispensable Guide to Eating the Right Carbs for Losing Weight and Optimum Health. Marlowe and Company, 2002.
M. Ajaib, S. Fatima, S.H. Kamran, K.M. Khan, S. Perveen, S. Shah, J. Chem. Soc. Pak. 38, 1267 (2016)
M. Ajaib, M. Latif, S.H. Kamran, K.M. Khan, S. Perveen, S. Shah, A. Karim, J. Chem. Soc. Pak. 38, 313 (2016)
M.A. Abbasi, S.A.H. Shah, Aziz-ur-Rehman, S.Z. Siddiqui, G. Hussain, K.M. Khan, M. Ashraf, S.A. Ejaz, J. Chem. Soc. Pak. 39, 248 (2017).
H. King, M. Rewers, Diabetes Care 16, 57 (1993)
P.R. Flanagan, G.G. Forstner, Biochem. J. 173, 553 (1978)
S. Chiba, Bioscience, Biotechnol. Biochem. 61, 1233 (1997)
K.T. Kim, L.E. Rioux, S.L. Turgeon, Phytochemistry 9, 827 (2014)
A.R. Saltiel, C.R. Kahn, Nature 414, 799 (2001)
H. Sun, D. Wang, X. Song, Y. Zhang, W. Ding, X. Peng, X. Zhang, Y. Li, Y. Ma, R. Wang, J. Agric Food Chem. 65, 1574 (2017)
P.M. Heacock, S.R. Hertzler, J.A. Williams, B.W. Wolf, J. Am. Diet. Assoc. 105, 65 (2005)
S.D. Kim, Food Chem. 136, 297 (2013)
N. Jong-Anurakkun, M.R. Bhandari, J. Kawabata, Food Chem. 103, 1319 (2007)
J.J. DiNicolantonio, J. Bhutani, J.H. O’Keefe, Open Heart 2, 1 (2015)
M.A. Avery, C.S. Mizuno, A.G. Chittiboyina, T.W. Kurtz, H.A. Pershadsingh, Curr. Med. Chem. 15, 61 (2008)
B.R. Miller, H. Nguyen, C.J.H. Hu, C. Lin, Q.T. Nguyen, Am. Health Drug Benef. 7, 452 (2014)
A.D. Mooradian, J.E. Thurman, Drugs 57, 19 (1999)
M.M. Wang, G.L. Dou, D.Q. Shi, J. Heterocycl. Chem. 47, 939 (2010)
O.O. Ajani, Bangladesh. J. Pharmacol. 11, 716 (2016)
I. Monreal, M. Sánchez-Castellanos, K. Ramírez-Gualito, G. Cuevas, K.A. Espinoza, I.A. Rivero, J. Braz. Chem. Soc. 30, 124 (2019)
M. Asif, Int. J. Med. Chem. 2014, 1 (2014)
S.K. Pandey, A. Singh, A. Singh, Eur. J. Med. Chem. 44, 1188 (2009)
M.A. Mohamed, R.R. Ayyad, T.Z. Shawer, A.-M. Alaa, A.S. El-Azab, Eur. J. Med. Chem. 112, 106 (2016)
S. Allameh, M. Heravi, M. Hashemi, F. Bamoharram, Chin. Chem. Lett. 22, 131 (2011)
N.M.A. Gawad, H.H. Georgey, R.M. Youssef, N.A. El Sayed, Med. Chem. Res. 20, 1280 (2011)
G. Moussa, R. Alaaeddine, L.M. Alaeddine, R. Nassra, A.S. Belal, A. Ismail, A.F. El-Yazbi, Y.S. Abdel-Ghany, A. Hazzaa, Eur. J. Med. Chem. 144, 635 (2018)
F.M. Refaie, A.Y. Esmat, S.M.A. Gawad, A.M. Ibrahim, M.A. Mohamed, Lipids Health Dis. 4, 22 (2005)
Y.I. El-Gazzar, H.H. Georgey, S.M. El-Messery, H.A. Ewida, G.S. Hassan, M.M. Raafat, M.A. Ewida, H.I. El-Subbagh, Bioorg. Chem. 72, 282 (2017)
M. Redondo, J.G. Zarruk, P. Ceballos, D.I. Pérez, C. Pérez, A. Perez-Castillo, M.A. Moro, J. Brea, C. Val, M.I. Cadavid, Eur. J. Med. Chem. 47, 175 (2012)
S.T. Al-Rashood, G.S. Hassan, S.M. El-Messery, M.N. Nagi, E.S.E. Habib, F.A. Al-Omary, H.I. El-Subbagh, Bioorg. Med. Chem. Lett. 24, 4557 (2014)
G.G. Berest, O.Y. Voskoboynik, S.I. Kovalenko, O.M. Antypenko, I.S. Nosulenko, A.M. Katsev, O.S. Shandrovskaya, Eur. J. Med. Chem. 46, 6066 (2011)
M.L. Heil, N.D. Cosford, R. Ardecky, and J. Zou, Google Patents, U.S. Patent No. 9,598,402. Washington, DC: U.S. Patent and Trademark Office (2017).
Y. Kabri, N. Azas, A. Dumetre, S. Hutter, M. Laget, P. Verhaeghe, A. Gellis, P. Vanelle, Eur. J. Med. Chem. 45, 616 (2010)
H. Niaz, H. Kashtoh, J.A. Khan, A. Khan, M.T. Alam, K.M. Khan, S. Perveen, M.I. Choudhary, Eur. J. Med. Chem. 95, 199 (2015)
U. Salar, M. Taha, K.M. Khan, N.H. Ismail, S. Imran, S. Perveen, S. Gul, A. Wadood, Eur. J. Med. Chem. 122, 196 (2016)
K.M. Khan, F. Rahim, A. Wadood, N. Kosar, M. Taha, S. Lalani, A. Khan, M.I. Fakhri, M. Junaid, W. Rehman, Eur. J. Med. Chem. 81, 245 (2014)
F. Rahim, H. Ullah, M.T. Javid, A. Wadood, M. Taha, M. Ashraf, A. Shaukat, M. Junaid, S. Hussain, W. Rehman, Bioorg. Chem. 62, 15 (2015)
K. Javaid, S.M. Saad, S. Rasheed, S.T. Moin, N. Syed, I. Fatima, U. Salar, K.M. Khan, S. Perveen, M.I. Choudhary, Bioorg. Med. Chem. 23, 7417 (2015)
H. Kashtoh, S. Hussain, A. Khan, S.M. Saad, J.A. Khan, K.M. Khan, S. Perveen, M.I. Choudhary, Bioorg. Med. Chem. 22, 5454 (2014)
M. Taha, N.H. Ismail, S. Lalani, M.Q. Fatmi, S. Siddiqui, K.M. Khan, S. Imran, M.I. Choudhary, Eur. J. Med. Chem. 92, 387 (2015)
S. Hameed, F. Seraj, R. Rafique, S. Chigurupati, A. Wadood, A.U. Rehman, V. Venugopal, U. Salar, M. Taha, K.M. Khan, Eur. J. Med. Chem. 183, 1 (2019)
M. Adib, F. Peytam, M. Rahmanian-Jazi, S. Mahernia, H.R. Bijanzadeh, M. Jahani, M. Mohammadi-Khanaposhtani, S. Imanparast, M.A. Faramarzi, M. Mahdavi, Eur. J. Med. Chem. 155, 353 (2018)
A.I. Khodair, M.A. Alsafi, M.S. Nafie, Carbohydr. Res. 486, 107832 (2019)
W. Liu, Q. Zhang, F. Gong, Z. Cao, Y. Huo, J. Heterocycl. Chem. 52, 317 (2015)
H.X. Wang, H.Y. Liu, W. Li, S. Zhang, Z. Wu, X. Li, C.-W. Li, Y.M. Liu, B.Q. Chen, Med. Chem. Res. 28, 203 (2019)
Acknowledgements
The authors are thankful to the Pakistan Academy of Sciences, Pakistan, for providing financial support to Project No. (5-9/PAS/440).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wali, H., Anwar, A., Shamim, S. et al. Synthesis, in vitro, and in silico studies of newly functionalized quinazolinone analogs for the identification of potent α-glucosidase inhibitors. J IRAN CHEM SOC 18, 2017–2034 (2021). https://doi.org/10.1007/s13738-021-02159-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02159-2