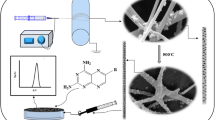
Abstract
In the present study, we utilized a novel electrochemical sensor for the determination of colchicine in 0.1 M phosphate buffer solution (pH 4.0) using differential pulse voltammetry. 1-butyl-3-methylimidazolium tetrachloroferrate ([Bmim]FeCl4) was synthesized, investigated by FT-IR, Raman and UV–Vis spectrometer and applied as magnetic ionic liquid in electrode modification. The other modifier was copper oxide nanoparticles/carbon nanofiber composite (CuO/CNF) provided by electrospinning and evaluated by scanning electron microscopy, X-ray diffraction and Fourier transform infrared spectroscopy (FT-IR). Several electro-analytical parameters such as the electrochemical behavior of the modified electrode, scan rate, type and supporting electrolyte pH were studied for the determination of colchicine. Under the optimized experimental conditions, oxidative peak current increased linearly by increasing colchicine concentration in the range of 1.0 to 100.0 nM and detection limit was obtained 0.25 nM. The relative standard deviation for six replicate analyses of 20.0 nM and 58.0 nM colchicine solution was obtained to be ± 3.17% and ± 2.08%, respectively. The proposed electrochemical sensor was successfully applied for colchicine quantification in colchicine tablet and health human plasma with satisfying results.












Similar content being viewed by others
References
A. Alamgir, Secondary metabolites: secondary metabolic products consisting of C and H; C, H, and O; N, S, and P elements; and O/N heterocycles, therapeutic use of medicinal plants and their extracts, vol. 2 (Springer, Berlin, 2018), pp. 165–309
D.M. Stanković, Ľ. Švorc, J.F. Mariano, A. Ortner, K. Kalcher, Electrochemical determination of natural drug colchicine in pharmaceuticals and human serum sample and its interaction with DNA. Electroanalysis 29(10), 2276–2281 (2017)
A. Slobodnick, B. Shah, M.H. Pillinger, S. Krasnokutsky, Colchicine: old and new. Am. J. Med. 128(5), 461–470 (2015)
A. Mandhare, P. Banerjee, Therapeutic use of colchicine and its derivatives: a patent review. Expert Opin. Ther. Pat. 26(10), 1157–1174 (2016)
M. Akodad, B. Lattuca, N. Nagot, V. Georgescu, M. Buisson, J.-P. Cristol, F. Leclercq, J.-C. Macia, R. Gervasoni, T.-T. Cung, COLIN trial: value of colchicine in the treatment of patients with acute myocardial infarction and inflammatory response. Arch. Cardiovasc. Dis. 110(6–7), 395–402 (2017)
G.J. Martínez, D.S. Celermajer, S. Patel, The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis 269, 262–271 (2018)
A.J. Stevenson, E.I. Ager, M.A. Proctor, D. Škalamera, A. Heaton, D. Brown, B.G. Gabrielli, Mechanism of action of the third generation benzopyrans and evaluation of their broad anti-cancer activity in vitro and in vivo. Sci. Rep. 8(1), 5144 (2018)
L. Liu, W. Zhang, L. Li, X. Zhu, J. Liu, X. Wang, Z. Song, H. Xu, Z. Wang, Biomechanical measurement and analysis of colchicine-induced effects on cells by nanoindentation using an atomic force microscope. J. Biomech. 67, 84–90 (2018)
D.A. Vrachatis, G. Giannopoulos, S.G. Deftereos, Colchicine: conventional and contemporary indications. Curr. Pharm. Des. 24(6), 647 (2018)
T. Hennessy, L. Soh, M. Bowman, R. Kurup, C. Schultz, S. Patel, G.S. Hillis, The low dose colchicine after myocardial infarction (LoDoCo-MI) study: a pilot randomized placebo controlled trial of colchicine following acute myocardial infarction. Am. Heart J. 215, 62–69 (2019)
M. Fu, J. Zhao, Z. Li, H. Zhao, A. Lu, Clinical outcomes after colchicine overdose: a case report. Medicine 98(30), e16580 (2019). https://doi.org/10.1097/MD.0000000000016580
W. Zhang, Q.-M. Zhou, G.-H. Du, Colchicine, natural small molecule drugs from plants (Springer, Berlin, 2018), pp. 503–507
M.R. Scipione, J. Papadopoulos, Pharmacokinetics and pharmacodynamics of antiviral drugs in special population, principles and practice of transplant infectious diseases (Springer, Berlin, 2019), pp. 977–1001
I. van Echteld, M.D. Wechalekar, N. Schlesinger, R. Buchbinder, D. Aletaha, Colchicine for acute gout. Cochrane Database Syst. Rev. 15(8), CD006190 (2014). https://doi.org/10.1002/14651858.CD006190.pub2
D.A. Moreira, F.M.D. Oliveira, D.M. Pimentel, T.J. Guedes, R. Luz, F.S. Damos, A.C. Pereira, R.A. da Silva, W.T. dos Santos, Determination of colchicine in pharmaceutical formulations and urine by multiple-pulse amperometric detection in an FIA system using boron-doped diamond electrode. J. Braz Chem. Soc. 29(9), 1796–1802 (2018)
N. Çankaya, İ. Bulduk, A.M. Çolak, Extraction, development and validation of HPLC-UV method for rapid and sensitive determination of colchicine from Colchicum autumnale L. Bulbs. Saudi J. Biol. Sci. 26, 345–351 (2018)
S. Bahrani, M. Ghaedi, K. Dashtian, A. Ostovan, M.J.K. Mansoorkhani, A. Salehi, MOF-5(Zn)-Fe2O4 nanocomposite based magnetic solid-phase microextraction followed by HPLC-UV for efficient enrichment of colchicine in root of colchicium extracts and plasma samples. J. Chromatogr. B 1067, 45–52 (2017)
S.A. Joshi, S.S. Jalalpure, A.A. Kempwade, M.R. Peram, Development and validation of HPLC method to determine colchicine in pharmaceutical formulations and its application for analysis of solid lipid nanoparticles. Curr. Pharm. Anal. 14(1), 76–83 (2018)
I. Capistrano, T. Naessens, L. Pieters, S. Apers, An HPLC method for the quantification of colchicine and colchicine derivatives in gloriosa superba seeds. Nat. Prod. Commun. 12, 1215–1221 (2017)
E. Abe, A.-S. Lemaire-Hurtel, C. Duverneuil, I. Etting, E. Guillot, P. de Mazancourt, J.-C. Alvarez, A novel LC-ESI-MS-MS method for sensitive quantification of colchicine in human plasma: application to two case reports. J. Anal. Toxicol. 30(3), 210–215 (2006)
S.W. Ng, C.K. Ching, A.Y.W. Chan, T.W.L. Mak, Simultaneous detection of 22 toxic plant alkaloids (aconitum alkaloids, solanaceous tropane alkaloids, sophora alkaloids, strychnos alkaloids and colchicine) in human urine and herbal samples using liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 942, 63–69 (2013)
M. Afzali, A. Mostafavi, T. Shamspur, Decoration of graphene oxide with NiO@ polypyrrole core-shell nanoparticles for the sensitive and selective electrochemical determination of piceatannol in grape skin and urine samples. Talanta 196, 92–99 (2019)
A. Khoshroo, L. Hosseinzadeh, A. Sobhani-Nasab, M. Rahimi-Nasrabadi, H. Ehrlich, Development of electrochemical sensor for sensitive determination of oxazepam based on silver-platinum core–shell nanoparticles supported on graphene. J. Electroanal. Chem. 823, 61–66 (2018)
L. El Harrad, I. Bourais, H. Mohammadi, A. Amine, Recent advances in electrochemical biosensors based on enzyme inhibition for clinical and pharmaceutical applications. Sensors 18(1), 164 (2018)
M. Afzali, A. Mostafavi, T. Shamspur, Designing an Au/reduced graphene oxide modified carbon paste electrode for the electrochemical quantification of agnuside. Sens. Actuators B Chem. 290, 188–194 (2019)
H. Bai, C. Wang, J. Chen, Z. Li, K. Fu, Q. Cao, Graphene@ AuNPs modified molecularly imprinted electrochemical sensor for the determination of colchicine in pharmaceuticals and serum. J. Electroanal. Chem. 816, 7–13 (2018)
Z. Jahromi, A. Mostafavi, T. Shamspur, M. Mohamadim, Magnetic ionic liquid assisted single-drop microextraction of ascorbic acid before its voltammetric determination. J. Sep. Sci. 40(20), 4041–4049 (2017)
Z. Jahromi, T. Shamspur, A. Mostafavi, M. Mohamadi, Separation and preconcentration of hemin from serum samples followed by voltammetric determination. J. Mol. Liq. 242, 91–97 (2017)
T.E. Sintra, M. Nasirpour, F. Siopa, A.A. Rosatella, F. Gonçalves, J.A. Coutinho, C.A. Afonso, S.P. Ventura, Ecotoxicological evaluation of magnetic ionic liquids. Ecotoxicol. Environ. Saf. 143, 315–321 (2017)
K.T. Greeson, N.G. Hall, N.D. Redeker, J.C. Marcischak, L.V. Gilmore, J.A. Boatz, T.C. Le, J.R. Alston, A.J. Guenthner, K.B. Ghiassi, Synthesis and properties of symmetrical N, N′-bis (alkyl) imidazolium bromotrichloroferrate (III) paramagnetic, room temperature ionic liquids with high short-term thermal stability. J. Mol. Liq. 265, 701–710 (2018)
K.D. Clark, O. Nacham, J.A. Purslow, S.A. Pierson, J.L. Anderson, Magnetic ionic liquids in analytical chemistry: a review. Anal. Chim. Acta 934, 9–21 (2016)
M.N. Emaus, K.D. Clark, P. Hinners, J.L. Anderson, Preconcentration of DNA using magnetic ionic liquids that are compatible with real-time PCR for rapid nucleic acid quantification. Anal. Bioanal. Chem. 410, 1–10 (2018)
Z.L. Xie, D.S. Su, Ionic liquid based approaches to carbon materials synthesis. Eur. J. Inorg. Chem. 2015(7), 1137–1147 (2015)
R. Zhuang, F. Jian, K. Wang, A new binuclear Cd (II)-containing ionic liquid: preparation and electrocatalytic activities. J. Organomet. Chem. 694(22), 3614–3618 (2009)
H. Wang, R. Yan, Z. Li, X. Zhang, S. Zhang, Fe-containing magnetic ionic liquid as an effective catalyst for the glycolysis of poly (ethylene terephthalate). Catal. Commun. 11(8), 763–767 (2010)
Q. Zhao, H. Xie, H. Ning, J. Liu, H. Zhang, L. Wang, X. Wang, Y. Zhu, S. Li, M. Wu, Intercalating petroleum asphalt into electrospun ZnO/carbon nanofibers as enhanced free-standing anode for lithium-ion batteries. J. Alloy Compd. 737, 330–336 (2018)
L.A. Mercante, A. Pavinatto, L.E. Iwaki, V.P. Scagion, V. Zucolotto, O.N. Oliveira Jr., L.H. Mattoso, D.S. Correa, Electrospun polyamide 6/poly (allylamine hydrochloride) nanofibers functionalized with carbon nanotubes for electrochemical detection of dopamine. ACS Appl. Mater. Interfaces 7(8), 4784–4790 (2015)
M. Afzali, Z. Jahromi, R. Nekooie, Sensitive voltammetric method for the determination of naproxen at the surface of carbon nanofiber/gold/polyaniline nanocomposite modified carbon ionic liquid electrode. Microchem. J. 145, 373–379 (2019)
J. Ping, S. Ru, K. Fan, J. Wu, Y. Ying, Copper oxide nanoparticles and ionic liquid modified carbon electrode for the non-enzymatic electrochemical sensing of hydrogen peroxide. Microchim. Acta 171(1–2), 117–123 (2010)
S. Peng, L. Li, J.K.Y. Lee, L. Tian, M. Srinivasan, S. Adams, S. Ramakrishna, Electrospun carbon nanofibers and their hybrid composites as advanced materials for energy conversion and storage. Nano Energy 22, 361–395 (2016)
Q. Gao, Z. Yuan, L. Dong, G. Wang, X. Yu, Reduced graphene oxide wrapped ZnMn2O4/carbon nanofibers for long-life lithium-ion batteries. Electrochim. Acta 270, 417–425 (2018)
G. Qiao, L. Zhang, F. Qiao, L. Dai, Methods of treating and preventing gout and lead nephropathy, Google Patents (2018)
P.K. Kalambate, C.R. Rawool, S.P. Karna, A.K. Srivastava, Highly sensitive and selective determination of methylergometrine maleate using carbon nanofibers/silver nanoparticles composite modified carbon paste electrode. Mater. Sci. Eng. C 69, 453–461 (2016)
M. Roushani, B.Z. Dizajdizi, A. Salimi, A. Azadbakht, Preparation of modified glassy carbon electrode by the use of titanium oxide, copper and palladium nanoparticles and its application for the electrocatalytic and photelectrocatalytic reduction of hydrogen peroxide. J. Mater. Sci. Mater. Electron. 30(5), 5212–5221 (2019)
M. Roushani, B.Z. Dizajdizi, Development of nonenzymatic hydrogen peroxide sensor based on catalytic properties of copper nanoparticles/Rutin/MWCNTs/IL/Chit. Catal. Commun. 69, 133–137 (2015)
M. Roushani, K. Bakyas, B.Z. Dizajdizi, Development of sensitive amperometric hydrogen peroxide sensor using a CuNPs/MB/MWCNT-C60-Cs-IL nanocomposite modified glassy carbon electrode. Mater. Sci. Eng. C 64, 54–60 (2016)
D. Ye, G. Liang, H. Li, J. Luo, S. Zhang, H. Chen, J. Kong, A novel nonenzymatic sensor based on CuO nanoneedle/graphene/carbon nanofiber modified electrode for probing glucose in saliva. Talanta 116, 223–230 (2013)
J. Song, L. Xu, C. Zhou, R. Xing, Q. Dai, D. Liu, H. Song, Synthesis of graphene oxide based CuO nanoparticles composite electrode for highly enhanced nonenzymatic glucose detection. ACS Appl. Mater. Interfaces 5(24), 12928–12934 (2013)
S. Alim, A. Kafi, R. Jose, M.M. Yusoff, J. Vejayan, Enhanced direct electron transfer of redox protein based on multiporous SnO2 nanofiber-carbon nanotube nanocomposite and its application in biosensing. Int. J. Biol. Macromol. 114, 1071–1076 (2018)
M. Afzali, A. Mostafavi, T. Shamspur, Square wave voltammetric determination of anticancer drug flutamide using carbon paste electrode modified by CuO/GO/PANI nanocomposite. Arab. J. Chem. 13, 3255–3265 (2018)
C. Zhang, L. Su, L. Huang, J. Cheng, J. Li, H. Qin, A facile synthesis of Cu–MnO/CNF by electrospinning method and its electrochemical performance for supercapacitor application. Int. J. Electrochem. Sci. 14, 11358–11366 (2019)
R.E. Del Sesto, T.M. McCleskey, A.K. Burrell, G.A. Baker, J.D. Thompson, B.L. Scott, J.S. Wilkes, P. Williams, Structure and magnetic behavior of transition metal based ionic liquids. Chem. Commun. (2008). https://doi.org/10.1039/b711189d
N.M. Deraz, S.A. Shaban, S.M. Morsy, S.M. Desouky, O.H. Abd-Elkader, Environmentally evaluation for desulfurization of gas oil with [BMIM][FeCl4] based on catalytic ionic liquids at room temperature. J. Pure Appl. Microbiol. 7, 387–393 (2013)
F.A. Yassin, F.Y. El Kady, H.S. Ahmed, L.K. Mohamed, S.A. Shaban, A.K. Elfadaly, Highly effective ionic liquids for biodiesel production from waste vegetable oils. Egypt. J. Pet. 24(1), 103–111 (2015)
M. Guzman, M. Arcos, J. Dille, S. Godet, C. Rousse, Effect of the concentration of NaBH4 and N2H4 as reductant agent on the synthesis of copper oxide nanoparticles and its potential antimicrobial applications. Nano Biomed. Eng. 10(4), 392–405 (2018)
S. Aziz, R. Abdulwahid, M. Rasheed, O. Abdullah, H. Ahmed, Polymer blending as a novel approach for tuning the SPR peaks of silver nanoparticles. Polymers 9(10), 486 (2017)
P.K. Raul, S. Senapati, A.K. Sahoo, I.M. Umlong, R.R. Devi, A.J. Thakur, V. Veer, CuO nanorods: a potential and efficient adsorbent in water purification. RSC Adv. 4(76), 40580–40587 (2014)
N. Dhineshbabu, V. Rajendran, N. Nithyavathy, R. Vetumperumal, Study of structural and optical properties of cupric oxide nanoparticles. Appl. Nanosci. 6(6), 933–939 (2016)
X.-H. Zhang, S.-M. Wang, L. Jia, Z.-X. Xu, Y. Zeng, Electrochemical properties of colchicine on the PoPD/SWNTs composite-modified glassy carbon electrode. Sens. Actuators B Chem. 134(2), 477–482 (2008)
K. Morawska, T. Popławski, W. Ciesielski, S. Smarzewska, Electrochemical and spectroscopic studies of the interaction of antiviral drug Tenofovir with single and double stranded DNA. Bioelectrochemistry 123, 227–232 (2018)
J. Hirst, Elucidating the mechanisms of coupled electron transfer and catalytic reactions by protein film voltammetry. Biochim. Biophys. Acta (BBA) Bioenerg 1757(4), 225–239 (2006)
F. Wang, J. Zhou, Y. Liu, S. Wu, G. Song, B. Ye, Electrochemical oxidation behavior of colchicine on a graphene oxide-Nafion composite film modified glassy carbon electrode. Analyst 136(19), 3943–3949 (2011)
W. Wen, Y. Tan, H. Xiong, S. Wang, Voltammetric and spectroscopic investigations of the interaction between colchicine and bovine serum albumin. Int. J. Electrochem. Sci. 5, 232–241 (2010)
Acknowledgements
The authors would like to express their sincere appreciation to the founders of Shahid Bahonar University of Kerman, Mr. Alireza Afzalipour and his wife, Mrs. Fakhereh Saba, for their foresight and generosity in training future generations of doctors, engineers and scientists. In addition, the authors would like to acknowledge their thanks to Dr. Parviz Dabiri for his generous support for the research activities of the chemistry and nanolaboratories in Shahid Bahonar University of Kerman.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afzali, M., Mostafavi, A. & Shamspur, T. Sensitive detection of colchicine at a glassy carbon electrode modified with magnetic ionic liquid/CuO nanoparticles/carbon nanofibers in pharmaceutical and plasma samples. J IRAN CHEM SOC 17, 1753–1764 (2020). https://doi.org/10.1007/s13738-020-01894-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-01894-2