Abstract
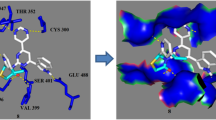
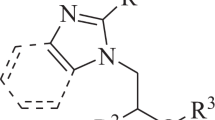
A series of imidazothiazole derivatives were synthesized via Claisen–Schmidt condensation of aldehyde 3, and different methyl ketones and their chemical structures were confirmed using 13C NMR, 1H NMR and LC–MS. In addition, the molecular structure of compound 3 was defined by single-crystal X-ray diffraction. The antibacterial and antifungal activities of synthesized compounds were investigated by diffusion method against three pathogenic bacteria (Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus) and one pathogenic fungus (Fusarium oxysporum). Compound 3 displayed significant antibacterial activity against E. coli and P. aeruginosa (MIC ≤ 0.2 mg/ml). Concerning the antifungal activity, all the molecules show very interesting results versus F. oxysporum (IC50 ≤ 0.07 mg/ml). These results were confirmed by the molecular docking studies such as some compounds showing optimum binding energy and affinity to the active site of the receptor.







Similar content being viewed by others
References
N.S. Shetty, I.A.M. Khazi, C. Ahn, Bull. Korean Chem. Soc. 31, 2337 (2010)
A. Andreani, M. Granaiola, A. Leoni, A. Locatelli, R. Morigi, M. Rambaldi, V. Garaliene, W. Welsh, S. Arora, G. Farruggia, J. Med. Chem. 48, 5604 (2005)
J. Robert, S. Boukraa, J. Panouse, V. Loppinet, J. Chaumont, Eur. J. Med. Chem. 25, 731 (1990)
G.D. Kapche, C.D. Fozing, J.H. Donfack, G.W. Fotso, D. Amadou, A.N. Tchana, M. Bezabih, P.F. Moundipa, B.T. Ngadjui, B.M. Abegaz, Phytochemistry 70, 216 (2009)
A. Kamal, D. Dastagiri, M.J. Ramaiah, J.S. Reddy, E.V. Bharathi, C. Srinivas, S. Pushpavalli, D. Pal, M. Pal-Bhadra, ChemMedChem 5, 1937 (2010)
O. Dahl, Ø. Fluge, E. Carlsen, J.N. Wiig, H.E. Myrvold, B. Vonen, N. Podhorny, O. Bjerkeset, T.J. Eide, T.B. Halvorsen, Acta Oncol. 48, 368 (2009)
A. Andreani, D. Bonazzi, M. Rambaldi, Arch. Pharm. 315, 451 (1982)
E.-J. Kim, J.-H. Kho, M.-R. Kang, S.-J. Um, Mol. Cell 28, 277 (2007)
C.B. Vu, J.E. Bemis, J.S. Disch, P.Y. Ng, J.J. Nunes, J.C. Milne, D.P. Carney, A.V. Lynch, J.J. Smith, S. Lavu, J. Med. Chem. 52, 1275 (2009)
R.M. Kumbhare, K.V. Kumar, M.J. Ramaiah, T. Dadmal, S. Pushpavalli, D. Mukhopadhyay, B. Divya, T.A. Devi, U. Kosurkar, M. Pal-Bhadra, Eur. J. Med. Chem. 46, 4258 (2011)
J.-H. Park, M.I. El-Gamal, Y.S. Lee, C.-H. Oh, Eur. J. Med. Chem. 46, 5769 (2011)
S. Koppireddi, D.R.K. Chilaka, S. Avula, J.R. Komsani, S. Kotamraju, R. Yadla, Bioorganic Med. Chem. Lett. 24, 5428 (2014)
F. Herencia, M.L. Ferrandiz, A. Ubeda, J. Domínguez, J.E. Charris, G.M. Lobo, M.J. Alcaraz, Bioorganic Med. Chem. Lett. 8, 1169 (1998)
N.U. Güzeldemirci, Ö. Küçükbasmacı, Eur. J. Med. Chem. 45, 63 (2010)
J.K. Malik, H. Soni, A. Singhai, J. Pharm. Res. 7, 39 (2013)
E. Gürsoy, N.U. Güzeldemirci, Eur. J. Med. Chem. 42, 320 (2007)
A. Andreani, M. Granaiola, A. Leoni, A. Locatelli, R. Morigi, M. Rambaldi, L. Varoli, D. Lannigan, J. Smith, D. Scudiero, Eur. J. Med. Chem. 46, 4311 (2011)
M. Allali, G.T. Benjelloun, N. Chahboun, Y. Mouacha, N. Allali, L. Bennani, I. Ouahidi, J. Ibijbijen, L. Nassiri, J. Mater. Environ. Sci. 8, 8 (2017)
R. Li, G.L. Kenyon, F.E. Cohen, X. Chen, B. Gong, J.N. Dominguez, E. Davidson, G. Kurzban, R.E. Miller, E.O. Nuzum, J. Med. Chem. 38, 5031 (1995)
E. Öhler, E. Zbiral, M. El-Badawi, Tetrahedron Lett. 24, 5599 (1983)
E.S. Hand, W. Paudler, Tetrahedron 38, 49 (1982)
B. Shivarama Holla, S. Ambekar, J. Indian Chem. Soc. 50, 673 (1973)
K. Kazauki, K. Htayama, S. Yokomor, T. Soki, Chem. Abstr. 85, 5913 (1976)
J.B. Cross, D.C. Thompson, B.K. Rai, J.C. Baber, K.Y. Fan, Y. Hu, C. Humblet, J. Chem. Inf. Model. 49, 1455 (2009)
C.R. Corbeil, C.I. Williams, P. Labute, J. Comput. Aided Mol. Des. 26, 775 (2012)
J.Y. Choi, M.S. Plummer, J. Starr, C.R. Desbonnet, H. Soutter, J. Chang, J.R. Miller, K. Dillman, A.A. Miller, W.R. Roush, J. Med. Chem. 55, 852 (2012)
S.C. Van, Verhandelingen-Koninklijke Academie voor Geneeskunde van Belgie 68, 223 (2006)
T.R. Shryock, Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: approved standard, NCCLC2002
Oxford Diffraction, CrysAlisPro (Oxford Diffraction Ltd, Abingdon, England, 2006)
Oxford Diffraction, CrysAlisPro (Oxford Diffraction Ltd, Abingdon, Oxfordshire, England, 2010)
A. Altomare, M.C. Burla, M. Camalli, G.L. Cascarano, C. Giacovazzo, A. Guagliardi, A.G. Moliterni, G. Polidori, R. Spagna, J. Appl. Crystallogr. 32, 115 (1999)
P.W. Betteridge, J.R. Carruthers, R.I. Cooper, K. Prout, D.J. Watkin, J. Appl. Crystallogr. 36, 1487 (2003)
D. Watkin, C. Prout, J. Carruthers, P. Betteridge, Crystals (Chemical Crystallography Laboratory, Oxford U.K., 1996)
Molecular Operating Environment Software (Chemical Computing Group Inc, Montreal, Canada, 2014)
ACD/ChemSketch (Advanced Chemistry Development, Inc, Toronto, ON, Canada, 2011). http://www.acdlabs.com
M.J. Dewar, E.G. Zoebisch, E.F. Healy, J.J. Stewart, J. Am. Chem. Soc. 107, 3902 (1985)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M.J. Bearpark, J. Heyd, E.N. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A.P. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N.J. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, Ö. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian 09 (Gaussian, Inc, Wallingford, 2009)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koudad, M., El Hamouti, C., Elaatiaoui, A. et al. Synthesis, crystal structure, antimicrobial activity and docking studies of new imidazothiazole derivatives. J IRAN CHEM SOC 17, 297–306 (2020). https://doi.org/10.1007/s13738-019-01766-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01766-4