Abstract
In this work, acetic acid functionalized poly(4-vinylpyridinum) bromide as a green and reusable catalyst was successfully tested on the synthesis of various bis-coumarins under solvent-free conditions. In addition, antioxidant and anti-inflammatory activities of the synthesized bis-coumarins were in vitro screened by 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging and formalin-induced edema model system, respectively. Antifungal activity was also evaluated against Fusarium oxysporum. Results showed that the synthesized bis-coumarins studied possess strong antioxidant (IC50; 0.149 ± 0.005 to 1.348 ± 0.006 mg/ml) and anti-inflammatory activities in comparison with ascorbic acid and diclofenac as positive controls, respectively. Also, the compounds showed good inhibition activity against Fusarium oxysporum (58 ± 1.4 to 100 ± 0.0%). For their biological activities, the synthesized bis-coumarins may be suggested to apply as biological agents for special use in future.
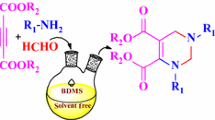
Graphical Abstract








Similar content being viewed by others
References
K. Tanaka, Solvent-Free Organic Synthesis (Wiley-VCH, GmbH and KGaA, Weinheim, 2004)
A. Khazaei, A.R. Moosavi-Zare, Z. Mohammadi, A. Zare, V. Khakyzadeh, G. Darvishi, RSC Adv. 3, 1323 (2013)
A. Khazaei, M.A. Zolfigol, A.R. Moosavi-Zare, F. Abi, A. Zare, H. Kaveh, V. Khakyzadeh, M. Kazem-Rostami, A. Parhami, H. Torabi-Monfared, Tetrahedron 69, 212 (2013)
A.R. Moosavi-Zare, M.A. Zolfigol, S. Farahmand, A. Zare, A.R. Pourali, R. Ayazi-Nasrabadi, Synlett 25, 193 (2014)
A.R. Moosavi-Zare, M.A. Zolfigol, M. Daraei, Synlett 25, 1173 (2014)
O.I. Aruoma, S.L. Cuppette, Champaign, vol. 142 (AOCS Press, Urbana, 1997)
M. Valko, D. Leibfritz, J. Moncol, Int. J. Biochem. Cell Biol. 39, 44 (2007)
L. Yu, J. Perret, B. Davy, J. Wilson, C.L. Melby, Food Chem. Toxicol. 67, 2600 (2002)
R.L. Prior, X.M. Wu, K. Schaich, J. Agric. Food Chem. 53, 4290 (2005)
M. Paya, B. Halliwell, J.R.S. Hoult, Biochem. Pharmacol. 44, 205 (1992)
A. Mora, M. Paya, J.L. Rios, M.J. Alcaraz, Biochem. Pharmacol. 40, 793 (1990)
S.A. Rice, M. Givskov, P. Steinberg, S. Kjelleberg, J. Mol. Microbiol. Biotechnol. 1, 23 (1999)
A. Ironmonger, B. Whittaker, J. Andrew, B. Baron, J. Chris, E. Alison, G. Ashcroft, A. Nelson, Org. Biomol. Chem. 5, 1081 (2007)
A.A. Al-Amiery, A. Mohammed, H. Ibrahim, A. Abbas, World Acad. Sci. Eng. Technol. 57, 433 (2009)
G. Kumar, D. Kumar, S. Devi, R. Verma, R. Johari, Int. J. Eng. Sci. Technol. 3, 1630 (2011)
A.H. Kadhum, A. Mohamad, A.A. Al-Amiery, Molecules 16, 6969 (2011)
R.D.H. Murray, J. Mendez, S.A. Brown, The Natural Coumarins (Wiley, Chichester, 1982)
A. Estevez-Braun, A.G. Ganzalez, Coumarins. Nat. Pdt. Rep. 14, 465 (1997)
M.A. Brimble, J.S. Gibson, J. Sperry, in Comprehensive Heterocyclic Chemistry III, ed. A.R. Katritzky, C.A. Ramsden, E.F. V. Scriven, R.J.K Taylor, (Elsevier, Amsterdam, 2008), vol 7, p. 557
R. Okenne, R.D. Thomes, Coumarins: Biology Application and Modes of Action (Wiley & Sons, Chichester, 1997)
M. Zahradink, The Production and Application of Fluorescent Brightening Agents (Wiley & Sons, New York, 1992)
R.S. Overmann, M.A. Stahmann, C.F. Heubner, W.R. Sullivan, L. Spero, D.G. Doherty, M. Ikawa, L. Graf, S. Rosenman, K.P. Lonk, J. Biol. Chem. 153, 5 (1944)
Z.H. Chohan, A.U. Shaikh, A. Rauf, C.T. Supuran, J. Enzym. Inhib. Med. Chem. 21, 741 (2006)
J. Jung, J.H. Lee, S. Oh, J.G. Lee, Bioorg. Med. Chem. Lett. 14, 5527 (2004)
Y.L. Chen, T.C. Wang, C.C. Tzeng, N.C. Chang, Helv. Chim. Acta 82, 191 (1999)
H. Zhao, N. Neamati, H. Hong, A. Mazumder, S. Wang, S. Sunder, G.W.A. Milne, Y. Pommier, T.R. Burke, J. Med. Chem. 40, 242 (1997)
V.D. Kancheva, P.V. Boranova, J.T. Nechev, I.I. Monolov, Biochimie 92, 1138 (2010)
A.N. Bhat, B.D. Jain, Talanta 5, 271 (1960)
G. Appendino, G. Cravotto, S. Tagliapietra, S. Ferraro, G.M. Nano, G. Palmisano, Helv. Chim. Acta 74, 1451 (1991)
I. Manolov, C.M. Moessmer, N. Danchev, Synthesis. Eur. J. Med. Chem. 4, 882 (2006)
J. Xu, J. Ai, S.H. Liu, X. Peng, L. Yu, M. Geng, F. Nan, Org. Biomol. Chem. 12, 3721 (2014)
G. Brufola, F. Fringuelli, O. Piermatti, F. Pizzo, Heterocycles 43, 1257 (1996)
J.S. Yadav, B.V.S. Reddy, A.K. Basak, B. Visali, A.V. Narsaiah, K. Nagaiah, Eur. J. Org. Chem. 3, 546 (2004)
A.V. Narsaiah, K. Nagaiah, Synth. Commun. 21, 3825 (2003)
P. Wasserscheid, W. Keim, Angew. Chem. Int. Ed. 39, 3772 (2000)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, Org. Prep. Proced. Int. 42, 95 (2010)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, J. Iran. Chem. Soc. 7, 646 (2010)
A. Khazaei, M.A. Zolfigol, A.R. Moosavi-Zare, A. Zare, Sci. Iran. Trans. C 17, 31 (2010)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, H.G. Kruger, Z. Asgari, V. Khakyzadeh, M. KazemRostami, J. Org. Chem. 77, 3640 (2012)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, V. Khakyzadeh, Appl. Catal. A 400, 70 (2011)
A. Khazaei, M.A. Zolfigol, A.R. Moosavi-Zare, A. Zare, E. Ghaemi, V. Khakyzadeh, Z. Asgari, A. Hasaninejad, Sci. Iran. Trans. C. 18, 1365 (2011)
M.A. Zolfigol, V. Khakyzadeh, A.R. Moosavi-Zare, A. Zare, S.B. Azimi, Z. Asgari, A. Hasaninejad, C. R. Chim. 15, 719 (2012)
A. Zare, T. Yousofi, A.R. Moosavi-Zare, RSC Adv. 2, 7988 (2012)
A. Khazaei, M.A. Zolfigol, A.R. Moosavi-Zare, Z. Asgari, M. Shekouhy, A. Zare, A. Hasaninejad, RSC Adv. 2, 8010 (2012)
M.A. Zolfigol, V. Khakyzadeh, A.R. Moosavi-Zare, G. Chehardoli, F. Derakhshan-Panah, A. Zare, O. Khaledian, Sci. Iran. Trans. C. 19, 1584 (2012)
A. Zare, A.R. Moosavi-Zare, M. Merajoddin, M.A. Zolfigol, T. Hekmat-Zadeh, A. Hasaninejad, A. Khazaei, M. Mokhlesi, V. Khakyzadeh, F. Derakhshan-Panah, M.H. Beyzavi, E. Rostami, A. Arghoon, R. Roohandeh, J. Mol. Liq. 167, 69 (2012)
A. Zare, T. Hekmat-Zadeh, S. Mirzaei-Monfared, M. Merajoddin, H. Torabi-Monfared, M.A. Zolfigol, A.R. Moosavi-Zare, E. Rostami, M. Mokhlesi, F. Derakhshan-Panah, S. Porbahiand, S. Balandeh, S. Afr, J. Chem. 65, 63 (2012)
A. Zare, F. Abi, A.R. Moosavi-Zare, M.H. Beyzavi, M.A. Zolfigol, J. Mol. Liq. 178, 113 (2013)
A.R. Moosavi-Zare, M.A. Zolfigol, M. Zarei, A. Zare, V. Khakyzadeh, J. Mol. Liq. 186, 63 (2013)
A. Khazaei, M.A. Zolfigol, A.R. Moosavi-Zare, J. Afsar, A. Zare, V. Khakyzadeh, M.H. Beyzavi, Chin. J. Catal. 34, 1936 (2013)
A.R. Moosavi-Zare, M.A. Zolfigol, M. Zarei, A. Zare, V. Khakyzadeh, A. Hasaninejad, Design. Appl. Catal. A. Gen. 467, 61 (2013)
A.R. Moosavi-Zare, M.A. Zolfigol, O. Khaledian, V. Khakyzadeh, Chin. J. Catal. 35, 573 (2014)
A.R. Moosavi-Zare, M.A. Zolfigol, O. Khaledian, V. Khakyzadeh, M. Darestani Farahani, H.G. Kruger, New J. Chem. 38, 2342 (2014)
A.R. Moosavi-Zare, M.A. Zolfigol, O. Khaledian, V. Khakyzadeh, M. Darestani Farahani, M.H. Beyzavi, H.G. Kruger, Chem. Eng. J. 248, 122 (2014)
A.R. Moosavi-Zare, M.A. Zolfigol, E. Noroozizadeh, O. Khaledian, B. Shirmardi Shaghasemi, Res. Chem. Intermed. 42, 4759 (2016)
A.R. Moosavi-Zare, M.A. Zolfigol, E. Noroozizadeh, M. Zarei, R. Karamian, M. Asadbegy, J. Mol. Catal. A: Chem. 425, 217 (2016)
M.A. Zolfigol, A.R. Moosavi-Zare, M. Zarei, C. R. Chimie. 17, 1264 (2014)
R. Karimian, F. Piri, A.A. Safari, S.J. Davarpanahm, J. Nanostruct Chem. 3, 1 (2013)
B. Karmakar, A. Nayak, J. Banerji, Tetrahedron Lett. 53, 4343 (2012)
D. Zavrsnik, S. Muratovic, D. Makuc, P. Plavec, M. Cetina, A. Nagl, E. De Clercq, J. Balzarini, M. Mintas, Molecules 16, 6023 (2011)
M. Hamdi, M.C. Puerta, P. Valerga, Eur. J. Med. Chem. 43, 2541 (2008)
J.R.S. Hoult, M. Paya, Gen. Pharmacol. 27, 713 (1996)
F. Borges, F. Roleira, N. Milhazes, L. Santana, E. Uriarte, Curr. Med. Chem. 12, 887 (2005)
R.M. Seabra, P.B. Andrade, P. Valentao, E. Fernandes, F. Carvalho, M.L. Bastos, M. Fingerman, R. Nagabhushanam, eds. (Science Publishers: Enfield, 2006), p. 115
C.A. Rice-Evans, N.J. Miller, G. Paganga, Antioxidant properties of phenolic compounds. Trends Plant Sci. 2, 152 (1997)
T.Y. Shen, in Burger’s Medicinal Chemistry, ed. M.E. Wolf (Wiley: New York, 1980) p. 1217
G.N. Agrios, 3rd ed. (Academic Press, Inc. New York, 1988), p. 803
R.R. Larkin, D.R. Fravel, Plant Diseases. 82, 1022 (1998)
J.E. Gray, P.R. Norton, R. Alnouno, C.L. Marolda, M.A. Valvano, K. Griffiths, Biomaterials 24, 2759 (2003)
S.A. Burt, Int. J. Food Microbiol. 94, 223 (2004)
L. Mensor, F.S. Menezes, G.G. Leitao, A.S. Reis, T.S. Santos, C.S. Coube, Phytotherapy Research 15, 127 (2001)
Acknowledgements
We thank Bu-Ali Sina University, Sayyed Jamaleddin Asadabadi University and Iran National Science Foundation (INSF) (Grant Number: 940124) for financial support to our research groups.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Noroozizadeh, E., Moosavi-Zare, A.R., Zolfigol, M.A. et al. Synthesis of bis-coumarins over acetic acid functionalized poly(4-vinylpyridinum) bromide (APVPB) as a green and efficient catalyst under solvent-free conditions and their biological activity. J IRAN CHEM SOC 15, 471–481 (2018). https://doi.org/10.1007/s13738-017-1247-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1247-1