Abstract
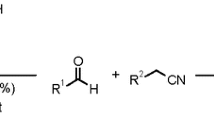
An efficient procedure has been reported for the synthesis of 4H-pyrano[2,3-c]pyrazoles in the presence of ZnS nanoparticles as a heterogeneous catalyst at the room temperature by grinding method. The ZnS nanoparticles were synthesized by hydrothermal method and characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy and N2 adsorption–desorption isotherm analysis. The 4H-pyrano[2,3-c]pyrazoles were obtained with high yields (87–97 %) in a short reaction time (5–21 min) under solvent-free condition. This method has advantages such as it is solvent free, uses simple grinding method that can be carried out at room temperature, requires short reaction times and production of pure products without any by product. The nanocatalyst can be easily recovered and reused for five runs without appreciable loss of its catalytic activity.






Similar content being viewed by others
References
G.A. Nawwar, F.M. Abdelrazek, R.H. Swellam, Arch. Pharm. (Weinheim) 324(11), 875 (1991)
V.Y. Sosnovskikh, M.A. Barabanov, B.I. Usachev, R.A. Irgashev, V.S. Moshkin, Russ. Chem. Bull. Int. Ed. 54(12), 2846 (2005)
T. Ueda, H. Mase, N. Oda, I. Ito, Chem. Pharm. Bull. (Tokyo) 29(12), 3522–3528 (1981)
L.J. Huang, M.J. Hour, C.M. Teng, S.C. Kuo, Chem. Pharm. Bull. (Tokyo) 40(9), 2547–2551 (1992)
P.T. Mistry, N.R. Kamdar, D.D. Haveliwala, S.K. Patel, J. Heterocycl. Chem. 49, 349 (2012)
A.H. Mandour, E.R. El-Sawy, M.S. Ebaid, S.M. Hassan, Acta. Pharm. 62, 15 (2012)
J.L. Wang, D. Liu, Z.J. Zheng, S. Shan, X. Han, S.M. Srinivasula, C.M. Croce, E.S. Alnemri, Z. Huang, Proc. Natl. Acad. Sci. USA 97, 7124 (2009)
M.E.A. Zaki, E.M. Morsy, F.M. Abdel-Motti, F.M.E. Abdel-Megeid, Heterocycl. Commun. 10, 97 (2004)
S.R. Mandha, S. Siliveri, M. Alla, V.R. Bommena, M.R. Bommineni, S. Balasubramanian, Bioorg. Med. Chem. Lett. 22, 5272 (2012)
D. Catarzi, L. Cecchi, V. Colotta, G. Filacchioni, C. Martini, P. Tacchi, A. Lucacchini, J. Med. Chem. 38(8), 1330 (1995)
A. Shaabani, A. Sarvary, A.H. Rezayan, S. Keshipour, Tetrahedron 65, 3492 (2009)
S.M. Yu, S.C. Kuo, L.J. Huang, S.S. Sun, T.F. Huang, C.M. Teng, J. Pharm. Pharmacol. 44(8), 667 (1992)
H.H. Otto, H. Schmelz, Arch. der Pharm. 312(6), 478 (1979)
A.M. Shestopalov, Y.M. Emeliyanova, A.A. Shestopalov, L.A. Rodinovskaya, Z.I. Niazimbetova, D.H. Evans, Org. Lett. 4, 423 (2002)
A.M. Shestopalov, Y.M. Emeliyanova, A.A. Shestopalov, L.A. Rodinovskaya, Z.I. Niazimbetova, D.H. Evans, Tetrahedron 59, 7491 (2003)
M. Bihani, P.P. Bora, G. Bez, J. Chem. (2013). doi:10.1155/2013/920719
M.B.M. Reddy, M.A. Pasha, Indian J. Chem. 51B, 537 (2012)
J. Ebrahimi, A. Mohammadi, V. Pakjoo, E. Bahramzade, A. Habibi, J. Chem. Sci. 124(5), 1013 (2012)
H.V. Chavan, S.B. Babar, R.U. Hoval, B.P. Bandgar, Bull. Korean Chem. Soc. 32(11), 3963 (2011)
Y. Peng, G. Song, R. Dou, Green. Chem. 8, 573 (2006)
D. Azarifer, S.M. Khatami, R. Nejat-Yami, J. Chem. Sci. 126(1), 95 (2014)
H. Kiyani, H.A. Samimi, F. Ghorbani, S. Esmaieli, Curr. Chem. Lett. 2, 197 (2013)
J.M. Khurana, A. Chaudhary, Green Chem. Lett. Rev. 5(4), 633 (2012)
H.G. Kathrotiya, R.G. Patel, M.P. Patel, J. Serb. Chem. Soc. 77(8), 983 (2012)
S.U. Tekale, S.S. Kauthale, K.M. Jadhav, R.P. Pawar, J. Chem. (2013). doi:10.1155/2013/840954
L. L. Chng, N. Erathodiyil, J. Y. Ying, Acc. Chem. Res. doi:10.1021/ar300197s
H. Naeimi, Z. Nazifi, J. Nanopart. Res. 15, 2026 (2013)
F. Zaera, Chem. Soc. Rev. 42, 2746 (2013)
S.S. Kahandal, S.R. Kale, M.B. Gawande, R.V. Jayaram, Catal. Sci. Technol. 4, 672 (2014)
R. Hudson, Y. Feng, R.S. Varma, A. Moores, Green Chem. 16, 4493 (2014)
S.N. Shelke, S.R. Bankar, G.R. Mhaske, S.S. Kadam, D.K. Murade, S.B. Bhorkade, A.K. Rathi, N. Bundaleski, O.M.N.D. Teodoro, R. Zboril, R.S. Varma, M.B. Gawande, ACS. Sustain. Chem. Eng. 2(7), 1699 (2014)
R.S. Varma, Sustain. Chem. Process. 2, 11 (2014)
G. Li, Z. Tang, Nanoscale 6, 3995 (2014)
Y. Imada, M. Ohsaki, M. Noguchi, T. Maeda, M. Fujiki, S. Kawamorita, N. Komiya, T. Naota, Chem. Cat. Chem. doi:10.1002/cctc.201402619
J. Yigang, W. Lei, F. Qinghua, Acta. Chim. Sin. 72(7), 798 (2014)
A. Dandia, V. Parewa, A.K. Jain, K.S. Rathore, Green Chem. 13, 2135 (2011)
E. Mohsen, J. Jaber, M.A. Mehdi, N.D. Fatemeh, J. Iran. Chem. Soc. 11, 499 (2014)
A.V. Borhade, B.K. Uphade, D.R. Tope, J. Chem. Sci. 125(3), 583 (2013)
A. V. Borhade, D. R. Tope, S. G. Gite, Arabian J. Chem. doi:10.1016/j.arabjc.2012.11.001
A.V. Borhade, B.K. Uphade, D.R. Tope, Res. Chem. Intermed. 40(1), 211 (2014)
A. V. Borhade, B. K. Uphade, A. G. Gadhave, In press. Res. Chem. Intermed. doi:10.1007/s 11164-013-1284-z
A.V. Borhade, D.R. Tope, D.R. Patil, J. Chem. Pharm. Res. 4(5), 2501 (2012)
Acknowledgments
The authors are thankful to CSIR, New Delhi for the financial assistance (01/2745/13/EMR-II) and Savitribai Phule Pune University as well as Sophisticated Analytical Instrument Facility (SAIF), Punjab University, Chandigarh for providing spectral analysis facilities. Authors are also thankful to Dr. Anil G. Gadhave for his helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borhade, A.V., Uphade, B.K. ZnS nanoparticles as an efficient and reusable catalyst for synthesis of 4H-pyrano[2,3-c]pyrazoles. J IRAN CHEM SOC 12, 1107–1113 (2015). https://doi.org/10.1007/s13738-014-0571-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-014-0571-y