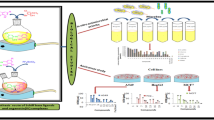
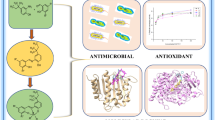
Abstract
Eight different di- and triorganotin(IV) complexes of vanillin-Schiff base of the type R2SnClL and R3SnL [R = Me (1,6), n-Bu (2,7), Ph (3,8), tert-Bu (4), Cy (5) and L = 4-((3,5-dimethylphenylimino)methyl)-2-methoxyphenol] were synthesized. The products were characterized by elemental analysis, FT-IR, 1H, 13C and 119Sn NMR spectroscopy. The ligand 4-((3,5-dimethylphenylimino)methyl)-2-methoxyphenol is also characterized by single crystal X-ray analysis. In the 1H NMR spectra of 1 and 6, the 2 J(119/117Sn-1H) in the Sn-CH3 moiety has 57 and 54 Hz values, respectively, that confirm the formation of four-coordinated Sn species. Moreover, the 13C NMR value for ipso carbon of SnPh is 139 ppm which also confirms the formation of four-coordinated Sn species. The title ligand and its complexes were also screened for their biological activities such as interaction with DNA, enzymatic, antimicrobial, cytotoxic and antioxidant activities. The antimicrobial and cytotoxic activities of triorganotin(IV) derivatives are relatively higher than their corresponding diorganotin(IV) analogues due to their greater lipophilicity and permeability through cell membrane.
Graphical abstract













Similar content being viewed by others
References
N. Galic, Z. Cimerman, V. Tomisic, Spectrochim. Acta Part A. 71, 1274 (2008)
W. Rehman, A. Badshah, S. Khan, L.T.A. Tuyet, Europ. J. Medical Chem. 44, 3981 (2009)
R.E. Hester, E.M. Nour, J. Raman Spectrosc. 11, 49 (1981)
E.M. Nour, A.A. Taha, I.S. Alnaimi, Inorg. Chim. Acta. 141, 139 (1988)
E.M. Nour, A.M. Al-Kority, S.A. Sadeek, S.M. Teleb, Synth. React. Inorg. Met. Org. Chem. 23, 39 (1993)
W. Wang, F.L. Zeng, X. Wang, M.Y. Tan, Polyhedron. 15, 1699 (1996)
L. Pellerito, L. Nagy, Coor. Chem. Rev. 224, 111 (2002)
S. Mishra, M. Goyal, A. Singh, Main Group Met. Chem. 25, 437 (2002)
A. Saxena, J.P. Tandon, A.J. Crowe, Polyhedron. 4, 1085 (1985)
A.D. Tiwari, A.K. Mishra, S.B. Mishra, B.B. Mamba, B. Maji, S. Bhattacharya, Spectrochim. Acta Part A. 79, 1050 (2011)
J.S. Casas, A. Castineiras, F. Condori, M.D. Couce, U. Russo, A. Sanchez, R. Seoane, Polyhedron. 22, 53 (2003)
R.G. Zarracino, J.R. Quinones, H. Hopfl, J. Organomet. Chem. 664, 188 (2002)
C. Pettiniari, F. Marchetti, R. Pettiniari, D. Martini, A. Drozdov, S. Troyanov, Synthesis and characterisation of tin(IV) and organotin(IV) derivatives 2-{[(2-hydroxyphenyl) imino]methyl}phenol. Inorg. Chim. Acta. 325, 103 (2001)
S. Tabassum, C. Pettinari, J. Organomet. Chem. 691, 1761 (2006)
M. Gielen, M. Biesemans, R. Willem, Appl. Organomet. Chem. 19, 440 (2005)
P. Yang, M. Guo, Coord. Chem. Rev. 185, 189 (1999)
A.J. Crowe, Antitumor activity of tin compounds, in Metal compounds in cancer therapy, ed. by S. P. Fricker (Chapman & Hall, London, 1994)
T.B. Baul, C. Masharing, G. Ruisi, R. Jirasko, M. Holcapek, D. de Vos, D. Wolstenholme, A. Linden, J. Organomet. Chem. 692, 4849 (2007)
H.D. Yin, S.W. Chen, L.W. Li, D.Q. Wang, Inorg. Chim. Acta. 360, 2215 (2007)
H.D. Yin, M. Hong, G. Li, D.Q. Wang, J. Organomet. Chem. 690, 3714 (2005)
M. Cagnoli, A. Alama, F. Barbieri, F. Novelli, C. Bruzzo, F. Sparatore, Anticancer Drugs 9, 603 (1998)
A.G. Davies, P.J. Smith, Adv. Inorg. Chem. Radiochem. 23, 1 (1980)
B.M. Elliot, W.N. Aldridge, J.M. Bridges, J. Biochem. 177, 461 (1979)
S.E.H. Etaiw, A.S. Sultan, A.S.B. El-din, Europ. J. Med. Chem. 46, 5370 (2011)
S.E.H. Etaiw, A.S. Sultan, A.S.B. El-din, M.M. El-bendary, Organomet. Chem. 696, 1668 (2011)
L.S. Lerman, J. Mol. Biol. 3, 18 (1961)
M.J. Waring, The molecular basis of antibiotic action (John Wiley and Sons, New York, 1972)
R.K. Gilpin, Anal. Chem. 69, 145R (1997)
Y.G. Jun, X.J. Juan, C.H. Yuan, L.Z. Zhou, Chin. J. Chem. 22, 1325 (2004)
M. Rodriguez, A.J. Bard, Anal. Chem. 62, 2658 (1990)
R. Barbieri, L. Pellerito, G. Ruisi, A. Silvestri, A. Barbieri Paulsen, G. Barone, S. Posante, M. Rossi, in Chemical processes in the marine environment, eds. by A. Gianguzza, E. Pelizzetti, S. Sammartano (Springer, Berlin, 2000)
P.J. Smith (ed.), Chemistry of Tin (Blackie Acad. and Prof., London, 1998)
W.L.F. Armarego, C.L.L. Chai, Purification of laboratory chemicals, 5th edn. (Butterworth-Heinemann, London, 2003)
C.V. Sastri, D. Eswaramoorthy, L. Giribabu, B.G. Maiya, J. Inorg. Biochem. 94, 138 (2003)
S. Dey, S. Sarkar, H. Paul, E. Zangrando, P. Chattopadhyay, Polyhedron. 29, 1583 (2010)
J. Ahlers, Biochem. J. 149, 535 (1975)
K. Jiao, W. Sun, H.Y. Wang, Chin. Chem. Lett. 13, 69 (2002)
K. Walter, C. Schütt, in Methods of enzymatic analysis, 2nd edn, ed. by H.U. Bergmeyer (Academic Press, New York, 1974)
M.M. Hossain, M.F. Aziz, R. Ahmed, M. Hossain, A. Mahmuda, T. Ahmed, M.H. Mazumder, Int. J. Pharm. Sci. 2, 60 (2010)
Z.A. Taha, A.M. Ajlouni, W. Al Momani, A.A. Al-Ghzawi, Spectrochim. Acta Part A. 81, 570 (2011)
A. Rehman, M.I. Choudhary, W.J. Thomsen, Bioassay techniques for drug development (Harwood Academic Publishers, Amsterdam, 2001)
B.N. Mayer, N.R. Ferrigni, J.E. Putnam, L.B. Jacobson, D.E. Nichols, J.L. Mclaughlin, Planta Med. 45, 31 (1982)
T. Sedaghat, S. Menati, Inorg. Chem. Commun. 7, 760 (2004)
A. Graisa, Y. Farina, E. Yousif, E.E. Saad, Intern. J. Chem. 1, 34 (2009)
S. Shahid, S. Ali, M. Hussain, M. Mazhar, S. Mahmood, S. Rehman, Turk. J. Chem. 26, 589 (2002)
M. Hanif, M. Hussain, S. Ali, M.H. Bhatti, M.S. Ahmed, B. Mirza, H.S. Evans, Turk. J. Chem. 31, 349 (2007)
J.S. Casas, A. Castineiras, F. Condori, M.D. Couce, U. Russo, A. Sanchez, R. Seoane, J. Sordo, J.M. Varela, Polyhedron. 22, 53 (2003)
C. Ma, Y. Han, R. Zhang, Inorg. Chim. Acta. 360, 2439 (2007)
V. Barba, E. Vega, R. Luna, H. Hopfl, I.H. Beltran, S.L. Zamudio-Rivera, J. Organomet. Chem. 692, 731 (2007)
A. Lycka, M. Nadvornik, K. Handlir, J. Holecek, Collect. Czech. Chem. Commun. 49, 2903 (1984)
J. Holecek, A. Lycka, D. Micak, L. Nagy, G. Vanko, J. Brus, S.S.S. Raj, H.K. Fun, S. Weng, Collect. Czech. Chem. Commun. 64, 1028 (1999)
T.P. Lockhart, W.F. Manders, J.J. Zuckerman, J. Am. Chem. Soc. 107, 4546 (1985)
T.P. Lockhart, W.F. Manders, Inorg. Chem. 25, 892 (1986)
T.P. Lockhart, W.F. Manders, J. Am. Chem. Soc. 109, 7015 (1987)
G. Casella, F. Ferrante, G. Saielli, Inorg. Chem. 47, 4796 (2008)
J. Holecek, M. Nadvornik, K. Handlir, A. Lycka, J. Organomet. Chem. 315, 299 (1986)
S.J. Blunden, P.A. Cussack, D.G. Gillies, Magn. Reson. Chem. 24, 921 (1986)
J. Holecek, A. Lycka, K. Handlir, M. Nadvornik, Collect. Czech Chem. Commun. 53, 571 (1988)
F.H. Allen, O. Kennard, D.G. Waston, L. Brammer, A.G. Orpen, R. Taylor, J. Chem. Soc. Perkin Trans. 2, S1 (1987)
A.A. Ensafi, R. Hajian, S. Ebrahimi, J. Braz. Chem. Soc. 20, 266 (2009)
R. Hajian, N. Shams, M. Mohagheghian, J. Braz. Chem. Soc. 20, 1399 (2009)
F.A. Shah, M. Sirajuddin, S. Ali, S.M. Abbas, M.N. Tahir, C. Rizzoli, Inorg. Chim. Acta. 400, 159 (2013)
M. Sirajuddin, S. Ali, A. Badshah, J. Photochem. Photobiol. B 124, 1 (2013)
M. Sirajuddin, S. Ali, N.A. Shah, M.R. Khan, M.N. Tahir, Spectrochim. Acta Part A. 94, 134 (2012)
M. Tariq, N. Muhammad, M. Sirajuddin, S. Ali, N.A. Shah, M.R. Khan, M.N. Tahir, J. Organomet. Chem. 723, 79 (2013)
M.R. Malik, V. Vasylyeva, K. Merz, N. Metzler-Nolte, M. Saleem, S. Ali, A.A. Isab, K.S. Munawar, S. Ahmad, Inorg. Chim. Acta. 376, 207 (2011)
F.J. Huoa, C.X. Yin, Y.B. Wu, P. Yang, Russ. J. Inorg. Chem. 55, 1087 (2010)
C.A. Kontogiorgis, D. Hadjipavlou-Litina, J. Enzyme Inhib, Med. Chem. 18, 63 (2003)
M.C. Foti, C. Daquino, C. Geraci, J. Org. Chem. 69, 2309 (2004)
A. Corona-Bustamante, J.M. Viveros-Paredes, A. Flores-Parra, A.L. Peraza-Campos, F.J. Martinez-Martinez, M.T. Sumaya-Martinez, A. Ramos-Organillo, Molecules 15, 5445 (2010)
S. Ahmad, S. Ali, S. Shahzadi, F. Ahmed, K.M. Khan, Turk. J. Chem. 15, 5445 (2010)
M.A. Neelakantan, M. Esakkiammal, S.S. Mariappan, J. Dharmaraja, T. Jeyakumar, Indian J. Pharm. Sci. 72, 216 (2010)
M.A. Neelakantan, F. Rusalraj, J. Dharmaraja, S. Johnsonraja, T. Jeyakumar, P.M. Sankaranarayana, Spectrochim. Acta A. 71, 1599 (2008)
M. Sirajuddin, S. Ali, A. Haider, N.A. Shah, A. Shah, M.R. Khan, Polyhedron. 40, 19 (2012)
M. Sirajuddin, S. Ali, Synthesis and characterization of potential bioactive organotins (LAP Lambert Academic Publishing, Germany, 2013)
Acknowledgments
M. Sirajuddin (Pin# P7-017) gratefully acknowledges the HEC Islamabad, Pakistan, for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material CRystallographic data for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre, CCDC# 814987 for HL. Copies of this information may be obtained free of charge from The Director, CCDC, 12, Union Road, Cambridge CB2 1EZ (FAX +44-1223-336033) or e-mail deposit@ccdc.cam. ac.uk or http://www.ccdc.cam.ac.uk.
Rights and permissions
About this article
Cite this article
Sirajuddin, M., Ali, S., Shah, F.A. et al. Potential bioactive Vanillin–Schiff base di- and tri-organotin(IV) complexes of 4-((3,5-dimethylphenylimino)methyl)-2-methoxyphenol: synthesis, characterization and biological screenings. J IRAN CHEM SOC 11, 297–313 (2014). https://doi.org/10.1007/s13738-013-0301-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-013-0301-x