Abstract
In this work, cloud point extraction (CPE) technique was developed for the separation and pre-concentration of Cd(II). CPE was used with lipophilic hexadentate (N4O2) Schiff base ligand, L22pysa (1, C24H26N4O2). The methodology is based upon the formation of a Cd(II)/L complex soluble in a micellar phase the non-ionic surfactant Triton X-114. This complex is then extracted into the surfactant-rich phase above its cloud point temperature. Several important variables that affect the CPE were investigated and optimized. Under the optimum experimental conditions, the calibration graph was linear over the range 1–100 ng mL−1 with a correlation coefficient of 0.9997. The detection limit obtained under optimum conditions was 0.44 ng mL−l. The proposed method was successfully applied to the determination of Cd(II) in rice and various water samples.
Similar content being viewed by others
Introduction
Cadmium is known as one of the most toxic elements among the heavy metals [1]. The effect of actuate cadmium poisoning in humans are high blood pressure, kidney damage and destruction of red blood cells [2]. Cadmium is mainly obtained as a byproduct in the hydrometallurgical processing of metals such as zinc, copper and lead. The residues from extraction of these metals contain considerable amounts of cadmium. Therefore, there is an increasing need to monitor cadmium levels in the environmental samples at ever decreasing concentrations. For this purpose, very sensitive, simple, rapid and inexpensive methods are necessary.
Flame atomic absorption spectrometry (FAAS) is usually used for cadmium determination [3]. However, it suffers from insufficient sensitivity for low concentrations of the metal in many environmental samples. Much effort has been expended to solve these problems; an enrichment and matrix elimination step is sometimes required before applying the above mentioned technique. Solvent extraction [4], ion exchange [5] and solid phase extraction [6] have been used for determination of low level concentrations of cadmium ions.
Cloud point extraction (CPE), known also as micelle-mediated phase separation extraction, is an attractive technique that reduces the consumption of and exposure to solvent, disposal costs and extraction time [7–14]. In general, the CPE of a metal ion takes place via a complex formation of the analyte with a lipophilic ligand. These complexes interact with the micellar aggregate and can be thus extracted from the aqueous solution into the surfactant-rich phase. The selectivity and efficiency of the method depend directly on the complexing ability of the lipophilic ligand. Schiff base ligands bearing nitrogen and oxygen donor atoms have been shown to exhibit interesting ionophoric properties, in particular toward heavy metal ions, and their applications in different separation and pre-concentration techniques have been investigated [15, 16]. For instance, Schiff base ligands are used as a modifier of octadecyl membrane disks for separation and concentration of trace amounts of metal ions [11]. These ligands have been also used in CPE methodology [15, 17]. In the present work, we show that the ligand L22pysa (Fig. 1) can be used as a complexing agent for the CPE of cadmium ions in water samples. Subsequent determination of cadmium by FAAS can then be accomplished.
Experimental
Reagents
The L22pysa ligand was synthesized according to the literature method [18] (see Fig. 1). Other chemical materials and reagents were of analytical grade and purchased from Merck (Darmstadt, Germany). A stock standard solution of cadmium at a concentration of 1,000 μg mL−1 was prepared from pure cadmium sulfate-8-hydrate. Working solutions were prepared by dilution of the stock solution. The acetate buffer pH 5 was prepared by adding NaOH solution to a 0.1 M acetic acid solution and adjusting pH to 5 using a pH meter.
Apparatus
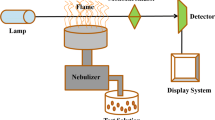
A Varian flame atomic absorption spectrometer (220AA) was used for metal ion determinations. The operating parameters were set as recommended by the manufacturer. Atomic absorption measurements were carried out in an air-acetylene flame. The following conditions were used: absorption line: Cd, 228.8 nm; slit widths, 0.6 nm; and lamp currents, 4 mA. The pH meter adjustments were performed using a Metrohm digital pH Meter (model 780) equipped with a combined glass electrode. A thermostated bath [Julabo (MP-5)] was used to control the temperature of CPE experiments. Phase separation was achieved using a centrifuge [Heraeus (Labofuge 300)]. A rotary shaker or magnetic stirrer [Heidolf (MR 3001)] was used to agitation.
Cloud point pre-concentration procedure
For the CPE, 10 mL of the sample solution pH 5 (acetic acid/sodium acetate buffer) containing the analyte, Triton X-114 0.06% (v/v) and ligand (6 × 10−6 M dissolved in methanol) was heated in a thermostated water bath at 40 °C for 20 min. Separation of two phases was accomplished by centrifugation for 10 min at 4,000 rpm. The sample was then cooled in an ice bath for 5 min to increase the viscosity of the surfactant-rich phase. The aqueous phase can readily be discarded by inverting the tube. A volume of 300 μL of nitric acid (0.5 M) in methanol was added to the surfactant-rich phase to reduce its viscosity and to facilitate sample handling prior to the FAAS assay. The final solution (500 μL) was introduced into the nebulizer of the spectrometer by conventional aspiration. This volume allows for achieving to a mean of two determinations by the FAAS instrument. In addition, the reported values are the mean value of at least two repeated CPE experiments. Calibration was performed using different standard solutions of cadmium, submitted to the same pre-concentration and determination procedures. Blank solution was also submitted to a similar procedure and measured in parallel to the sample solutions. To evaluate recovery, we used the equation where recovery (%) is defined as the ratio of A 1 to A 0.
Where A1 is the absorbtion after CPE, A0 is the absorbtion previous to CPE.
Sample preparation
Due to the biological and environmental significance of cadmium, three rice samples and several water samples were used to validate the accuracy of the proposed method. Rice samples were washed tree time with deionized water and dried in oven at 100 °C for 6 h. Then dried samples were crushed, ground and then sieved with a digestion procedure: 10 mL HNO3 (2 M) and 10 mL HCl (2 M) were added into vessels, in which exactly 0.2 g of the rice powder had been weighed and added. The pH was adjusted (pH 5) by addition NaOH solution (0.1 M). The mixture was heated and agitated on a rotary shaker (150 rpm, for 5 h).
Results and discussion
Effect of pH
The effect of pH upon the extraction of cadmium ions was the first parameter examined. Given a cation exchange mechanism for the complex formation of cadmium ion with L22pysa, the extraction yield will depend on the pH of the aqueous phase. Cloud point extraction of cadmium ions was therefore studied in the pH range of 1–7. Depending on the desired pH, the solutions were buffered by mixtures of phosphoric acid, formic acid or acetic acid with sodium hydroxide (all 0.1 M). The results illustrated in Fig. 2 show that at lower and higher pH values, the complex does not form completely, so that the extraction efficiency of cadmium is low. The decrease in the extraction at pH > 6 is probably due to the precipitation of cadmium as cadmium hydroxide, whereas, the decrease in extraction at pH < 2 is due to protonation of the ligand. Therefore, pH 5 was selected as the working pH value.
Effect of the chelating agent concentration
The concentration of the ligand was studied to determine its effect on the efficiency of extraction of cadmium ions. For this investigation, 10 mL of a solution containing 4 × 10−7 M cadmium in the presence of 0.06% (v/v) Triton X-114 buffered at pH 5 in the presence of different amounts of the Schiff base was subjected to the CPE process. The results are presented in Fig. 3. It can be seen that 6 × 10−6 M of Schiff base is selected as a suitable concentration of ligand for the extraction process.
Effect of the surfactant and its concentration
In CPE, an appropriate surfactant is important, since the temperature corresponding to the cloud point is correlated with the hydrophilic property of the surfactant. A successful CPE should therefore maximize the extraction efficiency by minimizing the phase volume ratio (V org/Vaq), thus increasing its concentrating capability. In this work, Triton X-114 was used because the CPT (cloud point temperature) of this compound is confirmed (25 °C) with room temperature. It should be noted that the above work was done in the room temperature.
At lower concentrations the extraction efficiency of cadmium complex is low. At higher concentrations the viscosity of the surfactant rich phase is increased leading to decreased aspiration rate and hence lower analytical signal. Therefore, concentration of 0.06% (v/v) TritonX-114 was selected for subsequent experiments (Fig. 4).
Effects of equilibration temperature and time
It was desirable to employ the shortest equilibration time and the lowest possible equilibration temperature, as a compromise between completion of extraction and efficient separation of phases. The dependence of extraction recovery upon equilibration temperature and time was studied over the temperature range 25–50 °C and over 5–25 min timeframes, respectively. Increasing the temperature to higher than 40 °C can decompose Schiff base. The results showed that an equilibration temperature of 40 °C and a time of 20 min were adequate to achieve quantitative extraction. The results are shown in Figs. 5 and 6, respectively.
Effects of foreign ions
In view of the high selectivity provided by FAAS, the interferences studied were those related to the pre-concentration step. To perform this study, 10 ml of solution containing 4 × 10−7 M cadmium and the interfering ion in different ion-to-analyte ratios was subjected to the extraction procedure. The tolerance limit was defined as the amount of foreign species causing a change in the absorbance of less than ±5%. The results are shown in Table 1 and these results allow the interference-free determination of cadmium in some environmental samples. The metal ions used were all in the chloride, nitrate or sulfate form.
Figure of merit
Calibration curves were obtained by pre-concentrating 10 ml of standard solution with Triton X-114. Table 2 features the analytical characteristics of the method. Under the optimal conditions, the calibration curves were linear up to 100 ng mL−1 for cadmium. The pre-concentration factor was calculated as the ratio of the aqueous solution volume (10 mL) to that of the surfactant rich phase volume after dilution with nitric acid (0.5 mL). The precision of the method (0.99%), calculated using the relative standard deviation (RSD) for five replicate experiments on the sample solutions of cadmium. The limit of detection (LOD) calculated as three times the standard deviation of the blank signal.
For comparison, the analytical features of the proposed method and those previously reported methods involving cadmium determination [19–22] are given in Table 3. It is seen that the LOD and the linear range of the proposed method in this work is superior to that of the most other reported methods. However, the short incubation time and lower incubation temperature are the additional advantages of the proposed method.
Applications
To validate the proposed methodology the developed procedure was applied to the determination of cadmium in several samples of Iranian rice and water. After digestion, the rice samples were subjected to CPE pre-concentration and subsequent instrumental measurements. The results are shown in Tables 4 and 5.
Conclusions
In this work, the use of micellar systems for separation and pre-concentration of cadmium offers several advantages including low cost, rapidity, safety, pre-concentration of cadmium with high recovery and very good extraction efficiency. The surfactant-rich phase can be easily introduced into the nebulizer of the spectrometer after dilution with 0.5 M HNO3 and directly determined by FAAS. The accuracy of the pre-concentration system was evaluated by recovery measurements on spiked several samples of Iranian rice and water.
References
Ulmann’s encyclopedia of industrial chemistry (Wiley-VCH, Weinheim, 2003)
K. Robards, P. Worsfold, Analyst 116, 549 (1991)
Welz B (1985) Atomic absorption spectroscopy. VCH, Amsterdam
R. Saran, T.S.B. Baul, R. Srinivas, D.T. Khathing, Anal Lett 25, 1545 (1992)
M. Shamsipur, A.R. Ghiasvand, H. Sharghi, H. Naeimi, Anal Chim Acta 408, 271 (2000)
C. Duran, H. Basri Senturk, L. Elci, M. Soylak, M. Tufekci, J Hazard Mater 162, 292 (2009)
S.A. Kulichenko, V.O. Doroschuk, S.O. Lelyushok, Talanta 59, 767 (2003)
V.O. Doroschuk, S.O. Lelyushok, V.B. Ishchenko, S.A. Kulichenko, Talanta 64, 853 (2004)
R. Liang, Z. Wang, J. He Xu, W. Li, H. Qi, Sep Pur Technol 66, 248 (2009)
A. Sanz-Medel, M.C. Temprano, N.B. Ordel Garcia, M.R. Fernandez, Anal Chem 67, 2216 (1995)
S.A.M. Fathi, M.R. Yaftian, J Colloid Interface Sci 334, 167 (2009)
J.L. Manzoori, H. Abdolmohammad-Zadeh, M. Amjadi, Talanta 71, 582 (2007)
S. Shariati, Y. Yamini, J Colloid Interface Sci 298, 419 (2006)
P. Liang, H. Sang, Z. Sun, J Colloid Interface Sci 304, 486 (2006)
S.A.M. Fathi, M.R. Yaftian, J Hazard Mater 164, 133 (2009)
S. Oshima, N. Hirayama, K. Kubono, H. Kokusen, T. Honjo, Talanta 59, 867 (2003)
F. Shemirani, M.R. Jamali, R.R. Kozani, M. Salavatiye-Niasari, Sep Sci Technol 41, 3065 (2006)
Salehzadeh S, Golbedaghi R (2007) J Chem Res 86
D. Zhao, R. Bian, Y. Ding, L. Li, J Iran Chem Res 2, 87–94 (2009)
J.L. Manzoori, A. Bavili-Tabrizi, Anal Chim Acta 470, 215–221 (2002)
S. Candir, I. Narin, M. Soylak, Talanta 77, 289–293 (2008)
C.B. Ojeda, F.S. Rojas, J.M.C. Pavón, Am J Anal Chem 1, 127–134 (2010)
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Golbedaghi, R., Jafari, S., Yaftian, M.R. et al. Determination of cadmium(II) ion by atomic absorption spectrometry after cloud point extraction. J IRAN CHEM SOC 9, 251–256 (2012). https://doi.org/10.1007/s13738-011-0018-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-011-0018-7