Abstract
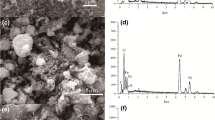
Nowadays, development of materials based on biopolymers in sorption process for removal of heavy metal ions from wastewater has been considered by many researchers. In this research, the nanogel of chitosan-g-thiacalix[4]arene (CS-g-TC4A) was synthesized from chitosan-azide and monopropargyl thiacalix[4]arene via click reaction. Firstly, Fe3O4-contained chitosan was synthesized via co-precipitation of Fe(II) and Fe(III) salts in the presence of chitosan. Then, magnetic chitosan grafted thiacalix[4]arene (Fe3O4-contained CS-g-TC4A) super-paramagnetic nanocomposites were prepared via click reaction of freshly prepared magnetic chitosan-azide with monopropargyl thiacalix[4]arene. The structure and surface morphology, thermal and magnetic properties of the nanogel and nanocomposite were characterized by Fourier transform infrared (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), thermal gravimetric analysis (TGA), atomic force microscopy (AFM) and vibrating sample magnetometer (VSM), respectively. The trend of heavy metal sorption in the CS-g-TC4A nanogel and Fe3O4-containing CS-g-TC4A super-paramagnetic nanocomposites is in the order of Pb(II) > Cd(II) > Co(II) > Ni(II) > Cu(II) > Cr(III) and Pb(II) > Cd(II) > Cu(II) > Ni(II) > Co(II) > Cr(III), respectively, in aqueous solutions at pH 7. The experimental data for all the cations were analyzed by isotherms and kinetics equations. The results of adsorption isotherm of cations on CS-g-TC4A nanogel and Fe3O4-contained CS-g-TC4A super-paramagnetic nanocomposite were fitted well with the Freundlich and Langmuir models, respectively. The maximal adsorption capacities of Pb(II), Cd(II), Co(II), Ni(II), Cu(II), Cr(III) by CS-g-TC4A nanogel and Fe3O4-contained CS-g-TC4A super-paramagnetic nanocomposite, as calculated from the Langmuir model, were 23.25, 16.20, 14.08, 13.88, 12.37, 11.11; and 23.28, 20.08, 15.89, 16.12,17.85, 14.92 mg/g, respectively. The kinetic study was in best agreement with pseudo-first-order model for both adsorbents.









Similar content being viewed by others
References
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418
Wan Ngah WS, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83:1446–1456
Karami H (2013) Heavy metal removal from water by magnetite nanorods. Chem Eng J 219:209–216
Tirtom VN, Dinçer A, Becerik S, Aydemir T, Çelik A (2012) Comparative adsorption of Ni(II) and Cd(II) ions on epichlorohydrin crosslinked chitosan–clay composite beads in aqueous solution. Chem Eng J 197:379–386
Kafshgari MH, Khorram M, Mansouri M, Samimi A, Osfouri S (2012) Preparation of alginate and chitosan nanoparticles using a new reverse micellar system. Iran Polym J 21:99–107
Sadeghi-Kiakhani M, Arami M, Gharanjig K (2013) Application of a biopolymer chitosan-poly(propylene)imine dendrimer hybrid as an antimicrobial agent on the wool fabrics. Iran Polym J 22:931–940
Tabakci M, Yilmaz M (2008) Synthesis of a chitosan-linked calix[4]arene chelating polymer and its sorption ability toward heavy metals and dichromate anions. Bioresour Technol 99:6642–6645
Li H, Zhan J, Chen M, Tian D, Zou Z (2009) Metal ions recognition by 1,2,3-triazolium calix[4]arene esters synthesized via click chemistry. J Incl Phenom Macrocycl Chem 66:43–47
Aksoy T, Erdemir S, Yildiz HB, Yilmaz M (2012) Novel water-soluble calix[4, 6]arene appended magnetic nanoparticles for the removal of the carcinogenic aromatic amines. Water Air Soil Pollut 223:4129–4139
Lakouraj MM, Tashakkorian H (2013) Synthesis of nanocrystalline polycalix[4]amides containing mesogenic triazole units and investigations of their thermo physical properties and heavy metal sorption behavior. J Macromol Sci Part A 50:310–320
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211–212:317–331
Hritcu D, Dodi G, Silion M, Popa N, Popa MI (2011) Composite magnetic chitosan microspheres: in situ preparation and characterization. Polym Bull 67:177–186
Zhang L, Zhu X, Sun H, Chi GR, Xu JX, Sun YL (2010) Control synthesis of magnetic Fe3O4–chitosan nanoparticles under UV irradiation in aqueous system. Curr Appl Phys 10:828–833
Yuwei C, Jianlong W (2011) Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu(II) removal. Chem Eng J 168:286–292
Gruškienė R, Čiuta G, Makuška R (2009) Grafting of poly (ethylene glycol) to chitosan at c (6) position of glucosamine units via “click chemistry” reactions. Chemija 20:241–249
Binder WH, Sachsenhofer R (2007) “Click” chemistry in polymer and materials science. Macromol Rapid Commun 28:15–54
Peng P, Cao X, Peng F, Bian J, Xu F, Sun R (2012) Binding cellulose and chitosan via click chemistry: synthesis, characterization, and formation of some hollow tubes. J Polym Sci Part A Polym Chem 50:5201–5210
Marmuse L, Nepogodiev SA, Field RA (2005) “Click chemistry” en route to pseudo-starch. Org Biomol Chem 3:2225–2227
Vesterinen A, Pahimanolis N, Seppala J, Rich J (2010) Modification of dextran using click-chemistry approach in aqueous media. Carbohydr Polym 82:78–82
Iki N, Kabuto C, Fukushima T, Kumagai H, Takeya H, Miyanari S, Miyashi T, Miyano S (2000) Synthesis of p-tert-butylthiacalix [4] arene and its inclusion property 1. Tetrahedron 56:1437–1443
Li Y, Hu X, Song X, Sun T (2012) Remediation of cadmium-contaminated soil by extraction with para-sulphonato-thiacalix[4]arene, a novel supramolecular receptor. Environ Pollut 167:93–100
Garg B, Bisht T, Chauhan S (2010) Ionic interactions of anionic thiacalix arene with cationic porphyrins. Arkivoc 2010:161–178
Lau YH, Rutledge PJ, Todd MH (2011) Chemical sensors that incorporate click-derived triazoles. Chem Soc Rev 40:2848–2866
Jiao TF, Zhou J, Zhou JX, Gao LH, Xing YY, Li XH (2011) Synthesis and characterization of chitosan-based Schiff base compounds with aromatic substituent groups. Iran Polym J 20(2):123–136
Lakouraj MM, Zare EN, Moghadam PN (2014) Synthesis of novel conductive poly(p-phenylenediamine)/Fe3O4 nanocomposite via emulsion polymerization and investigation of antioxidant activity. Adv Polym Technol 33:21385 (1–7)
Osuna Y, Gregorio-Jauregui KM, Gaona-Lozano JG (2012) Chitosan-coated magnetic nanoparticles with low chitosan content prepared in one-step. J Nanomater 2012:1–7
Molinari R, Argurio P, Poerio T (2006) Ultrafiltration of polymer-metal complexes for metal ion removal from wastewaters. Macromol Symp 235:206–214
Patent US, Pearson RG (1963) Hard and soft acids and bases. J Am Chem So 265:3533–3539
Murugesan A, Ravikumar L, Sathya Selva Bala V, SenthilKumar P, Vidhyadevi T, Dinesh Kirupha S, Kalaivani SS, Krithiga S, Sivanesan S (2011) Removal of Pb(II), Cu(II) and Cd(II) ions from aqueous solution using polyazomethineamides: equilibrium and kinetic approach. Desalination 271:199–208
Shen W, Chen S, Shi S, Li X, Zhang X, Hu W, Wang H (2009) Adsorption of Cu(II) and Pb(II) onto diethylenetriamine-bacterial cellulose. Carbohydr Polym 75:110–114
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. J Appl Chem 3:38–45
Ge F, Li MM, Ye H, Zhao BX (2012) Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J Hazard Mater 211–212:366–372
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lakouraj, M.M., Hasanzadeh, F. & Zare, E.N. Nanogel and super-paramagnetic nanocomposite of thiacalix[4]arene functionalized chitosan: synthesis, characterization and heavy metal sorption. Iran Polym J 23, 933–945 (2014). https://doi.org/10.1007/s13726-014-0287-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-014-0287-y