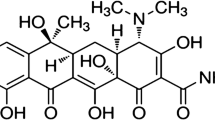
Abstract
Poly-ortho-aminophenol (PoAP) and multi-walled carbon nanotubes (MWCNTs) were deposited on the platinum electrode using cyclic voltammetry technique to form the Pt/PoAP/MWCNTs nanosensor for the electrochemical determination of oxytetracycline as analyte. This electrochemical nanosensor with good uniformity and high surface area was prepared in the presence of an ionic surfactant (sodium dodecyl sulfate) as electrolyte to suspend carbon nanotubes within the PoAP and improve the stability and electroactivity of the composite film. The surface morphology of the prepared nanosensor was characterized by scanning electron microscopy and showed a three-dimensional network structure. The influence of several parameters such as number of potential cycles, scan rate and pH of the solution on the electrochemical response of the resultant electrode was investigated. The prepared electrode functioned as a selective recognition element for oxytetracycline determination. It showed excellent electrochemical response to oxytetracycline at low oxidative potential in buffer solution of pH 2.0, with good stability and sensitivity. Under the optimal experimental conditions, the electrochemical response of the sensor was linear with respect to the concentration of oxytetracycline in a dynamic range of 0.2 μM–0.25 mM. The detection limit of the fabricated nanosensor was calculated as 0.10 μM (signal/noise = 3). This sensor was used successfully for the oxytetracycline determination in real samples with recoveries of 96.9–103.5 %.






Similar content being viewed by others
References
Wang HY, Park SM (2007) Polypyrrole-based optical probe for a hydrogen peroxide assay. Anal Chem 79:240–245
Pan X, Zhou S, Chen Ch, Kan J (2006) Preparation and properties of an uricase biosensor based on copolymer of o-aminophenol-aniline. Sens Actuators B Chem 113:329–334
Keyhanpour A, Seyed Mohaghegh SM, Jamshidi A (2012) Electropolymerization and characterization of polyaniline, poly(2-anilinoethanol) and poly(aniline-co-2-anilinoethanol). Iran Polym J 21:307–315
Li J, Shi L, An Y, Li Y, Chen X, Dong H (2006) Reverse micelles of star-block copolymer as nanoreactors for preparation of gold nanoparticles. Polymer 47:8480–8487
Liu L-P, Yin Zh-J, Yang Zh-Sh (2010) A l-cysteine sensor based on Pt nanoparticles/poly(o-aminophenol) film on glassy carbon electrode. Bioelectrochemistry 79:84–89
Li J, Zhao J, Wei X (2009) A sensitive and selective sensor for dopamine determination based on a molecularly imprinted electropolymer of o-aminophenol. Sens Actuators B Chem B 140:663–669
Chen Ch, Sun Ch, Gao Y (2009) Amperometric sensor for hydrogen peroxide based on poly(aniline-co-p-aminophenol). Electrochem Commun 11:450–453
Kan X, Zhou H, Li Ch, Zhu A, Xing Z, Zhao Zh (2012) Imprinted electrochemical sensor for dopamine recognition and determination based on a carbon nanotube/polypyrrole film. Electrochim Acta 63:69–75
Nabid MR, Sedghi R, Sharifi R, Abdi Oskooie H, Heravi MM (2013) Removal of toxic nitrate ions from drinking water using conducting polymer/MWCNTs nanocomposites. Iran Polym J 22:85–92
Pradhan AK, Swain SK (2013) Synthesis and characterization of poly(acrylonitrile-co-methylmethacrylate) nanocomposites reinforced by functionalized multiwalled carbon nanotubes. Iran Polym J 22:369–376
Benvidi A, Kakoolaki P, Zare HR, Vafazadeh R (2011) Electrocatalytic oxidation of hydrazine at a Co(II) complex multi-wall carbon nanotube modified carbon paste electrode. Electrochim Acta 56:2045–2050
Zamani MM, Fereidoon A, Sabet A (2012) Multi-walled carbon nanotube-filled polypropylene nanocomposites: high velocity impact response and mechanical properties. Iran Polym J 21:887–894
Schnappinger D, Hillen W (1996) Tetracyclines: antibiotic action, uptake, and resistance mechanisms. Arch Microbiol 165:359–369
Chopra I, Roberts M (2001) Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol R 65:232–260
Wang Y, Liu W-H, Wang K-M, Shen G-L, Yu R-Q (1998) Fluorescence optical fiber sensor for tetracycline. Talanta 47:33–42
Debuf Y (1988) The veterinary formulary. Pharmaceutical Press, London (97)
De Wasch K, Okerman L, De Brabander H, Van Hoof J, De Backer P (1998) Detection of residues of tetracycline antibiotics in pork and chicken meat: correlation between results of screening and confirmatory tests. Analyst 123:2737–2741
Jeon M, Paeng IR (2008) Quantitative detection of tetracycline residues in honey by a simple sensitive immunoassay. Anal Chim Acta 626:180–185
Croubels SM, Vanoosthuyze KE, Van Peteghem CH (1997) Use of metal chelate affinity chromatography and membrane-based ion-exchange as clean-up procedure for trace residue analysis of tetracyclines in animal tissues and egg. J Chromatogr B 690:173–179
Pena ALS, Lino CM, Silveira IN (1999) Determination of oxytetracycline, tetracycline, and chlortetracycline in milk by liquid chromatography with postcolumn derivatization and fluorescence detection. J AOAC Int 82:55–60
Pellegrini GE, Carpico G, Coni E (2004) Electrochemical sensor for the detection and presumptive identification of quinolone and tetracycline residues in milk. Anal Chim Acta 520:13–18
Monser L, Darghouth F (2000) Rapid liquid chromatographic method for simultaneous determination of tetracyclines antibiotics and 6-epi-doxycycline in pharmaceutical products using porous graphitic carbon column. J Pharm Biomed Anal 23:353–362
Ng M, Linder SW (2003) HPLC separation of tetracycline analogues: comparison study of laser-based polarimetric detection with UV detection. J Chromatogr Sci 41:460–466
Kowalski P (2008) Capillary electrophoretic method for the simultaneous determination of tetracycline residues in fish samples. J Pharm Biomed Anal 47:487–493
Han S, Liu E, Li H (2006) Determination of tetracycline, chlortetracycline and oxytetracycline by flow injection with inhibitory chemiluminescence detection using copper(II) as a probe ion. Luminescence 21:106–111
Pastor-Navarro N, Morais S, Maquieira Á, Puchades R (2007) Synthesis of haptens and development of a sensitive immunoassay for tetracycline residues application to honey samples. Anal Chim Acta 594:211–218
Jeon M, Kim J, Paeng K-J, Park S-W (2008) Biotin–avidin mediated competitive enzyme-linked immunosorbent assay to detect residues of tetracyclines in milk. Microchem J 88:26–31
Virolainen NE, Pikkemaat MG, Elferink JWA, Karp MT (2008) Rapid detection of tetracyclines and their 4-epimer derivatives from poultry meat with bioluminescent biosensor. J Agric Food Chem 56:11065–11070
Ehsani A, Mahjani MG, Jafarian M, Naeemy A (2010) An electrochemical study of the synthesis and properties of multi-walled carbon nanotube/poly-ortho-aminophenol composites. Prog Org Coat 69:510–516
Ojani R, Raoof J-B, Safshekan S (2009) Poly(o-aminophenol) film prepared in the presence of sodium dodecyl sulfate: application for nickel ion dispersion and the electrocatalytic oxidation of methanol and ethylene glycol. Electrochim Acta 54:2190–2196
Shaolin M (2004) Electrochemical copolymerization of aniline and o-aminophenol. Synth Met 143:259–268
Yang Ch-Sh, Wen T-Ch (1994) Electrochemical copolymerization of aniline para-phenylenediamine on IrO2-coated titanium electrode. J Appl Electrochem 24:166–178
Bard AJ, Faulkner LR (2001) Elecrochemical methods: fundamentals and applications, chap. 6. Wiley, New York
Lota G, Fic K, Frackowiak E (2011) Carbon nanotubes and their composites in electrochemical applications. Energ Environ Sci 4:1592–1605
Tucceri RI (2003) Specularity change on a thin gold film surface coated with poly(o-aminophenol) during the polymer redox conversion. The pH effect on the redox sites distribution at the metal–polymer interface. J Electroanal Chem 543:61–71
Acknowledgments
The support of this research by Payame Noor University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ajami, N., Bahrami Panah, N. & Danaee, I. Oxytetracycline nanosensor based on poly-ortho-aminophenol/multi-walled carbon nanotubes composite film. Iran Polym J 23, 121–126 (2014). https://doi.org/10.1007/s13726-013-0207-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-013-0207-6