Abstract
The expected lifespan of the world’s human population grows rapidly thanks to the great advance in modern medicine. While more and more body system diseases become treatable and curable, age-related neurodegenerative diseases remain poorly understood mechanistically, and are desperately in need of preventive and therapeutic interventions. Biomarker development consists of a key part of concerted efforts in combating neurodegenerative diseases. In many chronic neurodegenerative conditions, neuronal damage/death occurs long before the onset of disease symptoms, and abnormal proteolysis may either play an active role or be an accompanying event of neuronal injury. Increased spectrin cleavage yielding elevated spectrin breakdown products (SBDPs) by calcium-sensitive proteases such as calpain and caspases has been established in conditions associated with acute neuronal damage such as traumatic brain injury (TBI). In this article, we review literature regarding spectrin expression and metabolism in the brain, and propose a potential use of SBDPs as biomarkers for neurodegenerative diseases such as Alzheimer’s disease.
Similar content being viewed by others
Introduction
The spectrin family proteins are enriched membrane-associated molecules initially discovered in human red blood cells and then identified ubiquitously in various types of cells of bodily tissues, including the brain [1–4]. The most recent exciting discoveries in spectrin research includes the identification of essential biological functions of these proteins in the brain during neurodevelopment and synaptoplasticity, association of these proteins with particular neuronal structural domains, and findings of genetic link of these proteins to certain inherited neurological diseases such as spinocerebellar ataxia [3–7]. Potentially with a broader relevance in disease neurobiology, spectrin metabolism is intrinsically linked to membrane/cytoskeleton maintenance, dynamics, remodeling, and degradation, and also involves processes of protein cleavage, recycling, and clearance [6, 7]. In the event of neuronal stress, injury, and death under acute and chronic degenerative conditions, enhanced catabolism of membrane/cytoskeleton proteins occurs, leading to elevation of their breakdown products. Such a process provides a plausible rationale for seeking potential biomarkers for neurodegenerative disorders. Increased calpain- and caspase-3–mediated spectrin breakdown products (SBDPs) are found in a number of acute and subacute neurodegenerative conditions, including traumatic brain injury (TBI) [8–10]. Cerebrospinal fluid (CSF) and blood SBDPs are being evaluated as novel biomarkers for TBI in the clinic [11]. Ample evidence also exists suggestive of calpain/caspase-3 activation and enhanced spectrin proteolysis during brain aging and in Alzheimer’s disease (AD) and other age-related neurodegenerative disorders [12, 13].
In this review, we first will briefly introduce some general aspects of spectrin biology and the expression of brain spectrin. We then discuss spectrin proteolysis, especially by the calcium-dependent calpain and caspase-3 proteases as characterized in cell biology studies, and the development of SBDPs as novel biomarkers for TBI and cerebral ischemia. We will further review literature regarding calpain and caspase-3 activation and spectrin alterations during aging and in chronic neurodegenerative diseases, especially AD. Finally, we present preliminary observation of SBDP120 accumulation in transgenic AD model, and propose an application of SBDPs as potential biomarkers in age-related neurodegenerative diseases such as AD.
Overview of Spectrin Biology
Much of spectrin basic cell biology, subcellular localization, molecular interaction, functions, and disease relevance are learned from studies of the protein in red blood cells, which has been intensely discussed in many timely reviews [2–4, 14–18]. Overall, erythroid spectrin is composed of two monomeric components, namely α and β subunits, which connect each other laterally to form a dimmer that are further paired head-to-head to produce tetramers. The tetramers are then associated end-to-end with the short actin filaments to produce a basic hexagonal network located on the inner surface of red blood cell membrane. The β-spectrin subunits also contain binding sites for several other proteins, including ankyrin, protein 4.1, tropomyosin, tropomodulin, adducin, and glycophorin C [19–26]. The resulting spectrin meshwork complex appears to play an essential role in red blood cells to afford their morphological stability and flexibility and functions, including maintaining a biconcave morphology and changing shape in the circulation [17, 18]. The importance of erythroid spectrin complex in hematology has been implicated by the fact that genetic mutations of α-spectrin, β-spectrin, ankyrin, or protein 4.1 cause several forms of hereditary hemolytic anemia [4, 17, 18]. The spectrin membrane network is also expected to play a much broader biological role by providing orderly anchoring, trafficking, and dynamics of integral proteins within the lipid bilayer [3, 26, 29, 30].
A fundamental biological role of the spectrin family proteins is implicated by a conservative presence of the protein in bodily cells and during organism evolution. The spectrin gene family has expanded from invertebrates to vertebrates including mammals, with one α and two β genes coding spectrin subunits in invertebrates, whereas two α spectrins (αI and αII) and five β spectrins (βI to V) code the spectrin proteins in vertebrates including humans [4]. In humans, the SPTA1 gene encodes the αI-spectrin expressed in erythrocytes; its mutations result in hereditary disorders including elliptocytosis type 2, pyropoikilocytosis, and spherocytic hemolytic anemia [4, 15–17]. The SPTAN1 gene encodes αII-spectrin expressed specifically in nonerythrocytic cells including neurons, with its mutations associated with early infantile epileptic encephalopathy-5 [27]. The SPTB gene codes the erythroid β-spectrin subunit in humans [28], which is related to some form of hereditary spherocytosis as well [29]. The SPTBN1, SPTBN2, SPTBN4, and SPTBN5 genes appear to largely code the nonerythroid β-spectrin variants, named in order as βI-, βIII-, βIV-, and βV-spectrins, which are expressed in various tissues of the body [4, 16]. Mutations of some of the nonerythroid β-spectrin subunits could be associated with certain inherited diseases [30–34].
Spectrin Expression in the Nervous System
One of the most important extensions from the early erythroid spectrin research has been in the field of neuroscience, and brain spectrin perhaps represents the best-studied nonerythroid member of this protein family. In general, the brain appears to be considerably rich in spectrin, which is estimated to comprise about 3% of the total membrane protein [35, 36]. The major brain spectrin variants are the αII subunit and the βII, βIII, and βIV subunits, while it remains unclear if there is a differential expression of these subunits among neuronal/glial subpopulations [36, 37]. In immunoelectron microscopy, spectrin immunoreactive products are concentrated in neuronal cell bodies, dendrites, and postsynaptic terminals, with labeling prominently associated with the plasma membrane, microtubules, filaments, mitochondria, endoplasmic reticulum, and nuclear envelope [31, 38]. As expected, spectrin expression also has been identified in glial cells in the central and peripheral nervous systems, including oligodendrocytes, astrocytes, and Schwann cells [39]. As with their erythroid counterparts, brain spectrin subunits crosslink with actin filament and other proteins such as ankyrin, consisting with a major and general function of the protein in membrane skeleton formation, maintenance, and plasticity [4, 26, 36]. Importantly, brain spectrin appears to be “specialized” at certain neuronal compartments to serve unique neurobiological roles that involve special assembly of local membrane domain and/or dynamic membrane/cytoskeletal modulation. For instance, brain spectrin proteins participate in assembly of specialized membrane proteins at the initial axonal segment, Ranvier’s node, growth cone, and pre- and postsynaptic components [40–48]. Thus, neuronal and glial spectrin may play fundamental roles during membrane/cytoskeletal remodeling and rearrangement events (eg, neuronal development and migration, neuritic polarization and outgrowth, and synaptogenesis and plasticity) [40–53].
At least one hereditary neurological disorder, called spinocerebellar ataxia type 5 (SCA5), is associated with spectrin mutations with the βIII-spectrin gene (SPTBN2). This was first identified in a family descended from the grandparents of President Abraham Lincoln [54], and later in three other families [5, 54]. The disease is pathologically characterized by Purkinje cell degeneration. Subsequent experimental genetic studies in drosophila show that the mutant βIII-spectrin disrupts fundamental intracellular protein transportation [54]. In a mouse model lacking full-length βIII spectrin, animals recapitulate many clinical and pathological features of SCA5, including gait abnormalities, tremor, deteriorating motor coordination, and cerebellar atrophy [55]. Disruption of dendritic arborization and spine formation in Purkinje cells occurs in βIII-spectrin knockout mice during development. These defects appear largely to affect dendritic but not axonal development, suggesting a critical role of the spectrin protein in neuronal development [50, 52–55].
Spectrin Proteolysis in Neuronal Injury and Death
Spectrin catabolism would involve multiple events such as proteolysis, ubiquitination, intracellular trafficking/sequestration, and release/exocytosis of breakdown products [56, 57]. So far, the proteolytic process has been studied mostly under acute or subacute stressful conditions in vitro and in vivo. Both αII- and βII-spectrin proteins may undergo enzymatic cleavages by calpain and caspase-3 [58–62]. αII-Spectrin (280 kDa) is a major substrate for calpain and caspase-3 proteases, and can produce multiple breakdown products with distinct molecular sizes. Calpain-mediated degradation of αII-spectrin results in the formation of two unique and highly stable SBDPs migrated at 150 kDa and 145 kDa (SBDP150 and SBDP145), which can be further cleaved by caspase-3 yielding shorter fragments including SBDP120. The presence of the calpain-cleaved fragments occurs early in neural cell pathology and may be indicative of necrotic and excitotoxic neuronal injury and death. Caspase-3–mediated αII-spectrin degradation yields the formation of 150-kDa SBDP, which is further cleaved into a 120-kDa fragment (SBDP120) and appears to be indicative of apoptotic death. A number of antibodies are thus developed to detect different SBDPs, which may help determine cell death mediated by calpain and/or caspase cleavages and assess neuronal injury and death in vivo including in clinical setting [8–11, 58–62].
The causal relationship between intracellular calcium rise, calpain activation, and spectrin breakdown has been well-established in vivo and ex vivo (organotypic culture) in the hippocampal formation [63]. Thus, pharmacological stimulation of N-methyl-D-aspartate (NMDA) receptors activates calpain-dependent spectrin proteolysis, leading to elevations of SBDPs and signs of neuronal degeneration that both can be blocked by NMDA antagonists [64–66]. Similarly, calpain activation associated with increased neuronal SBDP immunoreactivity and cell loss occurs in the hippocampus and piriform cortex after kainate-induced excitotoxicity and seizure [66, 67]. Enhanced spectrin breakdown and/or elevation of SBDPs also occur in the other parts of the central nervous system as a result of hypoxia and ischemia. In ex vivo retinal preparations, hypoxia upregulates calpain and its mediated αII-spectrin proteolysis leading to increased levels of SBDP150 [68]. Both calpain- and caspase-3–specific SBDPs are elevated in the retina after intraocular hypertension, an experimental model of glaucoma [69]. In a typical gerbil model of global ischemia, transient (5 minute) carotid artery occlusion results in elevation of SBDP150 in the forebrain for up to 15 days [70].
The use of CSF SBDPs as biomarkers for neuronal injury is currently explored experimentally and clinically for a number of neurological conditions. Elevation of calpain- and caspase-specific SBDPs in CSF after TBI has been established in multiple human studies, and appears to be correlated with the severity of injury, computed tomography scan findings, and clinical outcomes [8, 11, 71, 72]. The temporal profiles of CSF SBDPs change appear of predictive value for clinical outcome, with persistent SBDP elevation associated with worse recovery. Levels of SBDP150 and SBDP145 are predominantly elevated during the early phase post-TBI, while SBDP120 levels remain elevated for a longer postinjury period. This pattern suggests that both necrotic/oncotic and apoptotic cell death mechanisms are activated after TBI, but may proceed with different time courses after injury [8, 71, 72]. Besides TBI, accumulation of calpain-cleaved αII-SBDPs are detectable in the CSF in rats subjected to 2 hours of transient focal cerebral ischemia by middle cerebral artery occlusion followed by reperfusion [61], and in a canine model of hypothermic circulatory arrest [9]. These findings point to a potential utility of CSF SBDPs as biomarkers for ischemic brain damages in addition to TBI in humans. One may expect that neuronal spectrin degradation by calcium-dependent proteases calpain and caspase-3 may occur under diverse conditions and potentially reflect an early but general event in neural cell pathology [7].
Neuronal Injury/Death Signaling in Chronic Neurodegenerative Diseases
While the underlying etiological cause, brain regional vulnerability, cellular neuropathology, and clinical manifestation are significantly different, many age-related neurodegenerative diseases including AD, Parkinson’s disease (PD), and Huntington’s disease (HD) appear to be associated with chronic neuronal stress, injury, and death, which are believed to begin and progress over a long period of time before the appearance of presenting clinical symptoms in each disease [73–76]. Neuronal degeneration in these chronic disease paradigms appears to exhibit many molecular and cellular characteristics similar to that seen during the course of acute neuronal injury and death after traumatic, ischemic, or other stressful insults. As well recognized in the field, calcium dyshomeostasis and calcium-induced activation of proteases, including calpain and caspases, may contribute to neuronal death in a wide variety of neurodegenerative diseases [74–76]. For example, increased calpain II (m-calpain) is found in vulnerable brain regions in PD [77]. Activation of the calpain/cdk5/p25 pathway is evident in the cingulate gyrus in PD [78]. In fact, calpain participates in α-synuclein cleavage and play a role in α-synuclein aggregation and pathogenesis [79]. Similarly, activated calpain has been detected in the caudate of human HD tissue but not in age-matched controls [80]. In fact, both calpain and caspse-3 are key proteases for huntingtin degradation [79, 80]. In AD, widespread calpain activation and the upregulation of the caspase signaling pathway appear to occur early and persistently in brain [81–84]. Again, the calcium-sensitive proteases are involved in the cleavages of some disease-signature proteins in AD, including β-amyloid precursor protein (APP) [85], β-secretase (BACE1) [86••], and tau [87]. Thus, calcium deficits and associated cell injury/death signaling activation are likely early and fundamental events in various neurodegenerative diseases, pointing to novel molecular targets of disease intervention as well as avenues for the development of diagnostic biomarkers.
Spectrin Proteolysis and SBDPs in Aging and Alzheimer’s Disease
A fair amount of evidence suggests that calcium deregulation and increased proteolysis of spectrins may exist in the brain during normal aging and in AD [12, 84, 85, 86••, 87–90]. In mouse and rat brains, levels of SBDPs elevate with age especially in the forebrain, largely involving the calpain-specific species SBDP150 and SBDP145 [91, 92]. Altered spectrin and SBDP150 immunoreactivity is reported in AD human brain [13], with abnormal spectrin labeling occurring in neuronal processes and axon terminals, which may represent atypical axonal and dendritic elements from sprouting neurons or accumulation of SBDPs in degenerating neurons. In another human brain study, accumulation of SBDP120 is found in AD but not age-matched control brains, predominantly localizing to a subpopulation of layers III and V cortical pyramidal neurons [93]. These findings are in congruence with existing anatomic and biochemical evidence indicating calpain overexpression and activation in this disease [84, 85, 86••, 87] . Potentially relevant to aging or AD cell biology, enhanced spectrin proteolysis and elevation of SBDPs can be detected in cultured skin fibroblasts from aged and AD donors as compared to young adult controls [12], and in red blood cells and lens during aging [89, 90].
Two recent studies have shown alteration of SBDPs in the CSF and/or brain in AD patients and transgenic mouse models of AD relative to control patients. Higuchi et al. [86••] analyzed CSF samples from 14 living AD patients and 9 age-matched normal control patients, showing that CSF levels of the 150- and 145-kDa SBDPs elevated over 2.5-fold in AD relative to control cases, with the ratio of cleaved to full-length species increased more than threefold in the former group. In 16-month-old mice expressing mutant human APP, levels of a calpain-cleaved α-spectrin (136 kDa) increased about tenfold in the hippocampal extracts relative to nontransgenic (non-Tg) control. In immunohistochemistry, putative SBDP150 labeling was identified in dystrophic neurites surrounding amyloid plaques [86••]. In another study, SBDP150 levels were found increased by western blot in the hippocampus in APP/presenilin-1 double-transgenic mice relative to non-Tg controls [94••]. Interestingly, blocking calpain activity by genetic overexpression of an endogenous calpain inhibitor, calpastatin, caused a remarkable decrease of amyloid plaque pathology by immunohistochemistry and also prevented tau phosphorylation in biochemical analysis [94••].
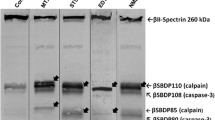
Using a set of new antibodies, we are currently examining potential SBDP accumulation in the brain during normal aging, and in transgenic models of AD relative to control by anatomical and biochemical approaches. Preliminary western blot data indicated elevations of SBDP150 and SBDP145 in aged rat and mouse brains, more robust in AD transgenic animals, consistent with a notion of calpain overexpression/activation facilitating spectrin cleavages. Interestingly, a rabbit antibody targeting the caspase-3 cleavage site in αII-spectrin detected SBDP120 by western blot, but displayed selective regional and cellular labeling in aged rat forebrain. In rats, SBDP120 protein levels elevated in the cerebral cortex and olfactory bulb by 16 months relative to 4 and 8 months of age (Fig. 1a, b). This antibody also revealed age-dependent but region- and cell-type–specific labeling in 16-month-old rats, occurring largely in layer V pyramidale-like cortical neurons and some dendritic elements (Fig. 1c, d), and distinctly at the apical dendrites of the olfactory bulb mitral cells (Fig. 1g, j, [arrows]). SBDP120 protein levels and intraneuronal immunoreactivity were increased age-dependently in the cerebral cortex and olfactory bulb in the triple transgenic AD (3xTg-AD) model mice relative to non-Tg counterparts (Cai et al., unpublished observations). Interestingly, in the transgenic mice that harbor five familial AD (FAD)–related mutations (5XFAD), which undergo an aggressive amyloid pathogenesis [95], robust astrocytic SBDP120 immunolabeling was detectable in many forebrain areas before middle age, whereas little astrocytic reactivity was seen in non-Tg controls. This finding is surprising, and it remains to be clarified whether glial cells may contribute to SBDP formation in the brain or they may uptake and further process SBDPs derived from neurons. Regardless, it appears that transgenic AD models may be useful for analyzing spectrin proteolysis and SBDP accumulation in the brain, which may be informative for future characterization of the pathology in AD.
Preliminary characterization of an age-related accumulation of SBDP120 in rodent brain with a new rabbit polyclonal antibody. a. The antibody detects a band at 120 kDa in rat forebrain extracts by western blot, a, b. with its banding signal increased in the cortex and olfactory bulb at 16 relative to 4 and 8 months of age, as also seen in c, d. immunolabeling in the cortex. e, f. SBDP120 immunoreactivity is substantially increased in the olfactory bulb (OB), cerebral cortex (Ctx), and hippocampal formation in an 8-month-old 5XFAD mouse relative to h, i. an age-matched nontransgenic (non-Tg) control. g. The labeling is strikingly expressed in astrocytic glia in the transgenics. However, neuronal labeling is readily evident in e, h, j the olfactory nerve terminals and around g, j. the mitral cell layer (MCL; shown by arrows). The latter labeling was identified to occur at the somatodendritic junction and apical dendrites of the mitral cells. Scale bar = 300 μm for c, d, g, i, and 1 mm for e, f, h and j. SBDP spectrin breakdown product; Pir piriform cortex; WM white matter; CA1 hippocampal CA1 sector; DG dentate gyrus; GCL granule cell layer; SEZ subependymal zone; GL glomerular layer; EPL external plexiform layer
SBDPS as Putative Biomarkers for Neurodegenerative Diseases
As discussed in preceding sections, spectrin proteins are enriched constitutive cellular components that play vital biological roles in membrane/cytoskeleton stability, dynamics, and remodeling. In the brain, such structural cellular modulations are constant and essential to support neuronal and synaptic plasticity, mediated at least in part by activity-dependent calcium signaling that activates effector proteases, including calpain and caspases, to execute site-specific and temporal degradation of specific proteins such as spectrin, allowing membrane/cytoskeleton reassembly to take place [6, 7]. Aberrant activation of calpain and caspases by calcium overflow is an early cellular response to many stressful and potentially pathogenic insults, which, if persistent, can lead to membrane/cytoskeleton destruction and death in vulnerable populations of cells. As established in traumatic and ischemic brain injuries, calcium triggered calpain and caspases-3 activation cause excessive spectrin proteolysis and subsequent elevation of SBDPs in the brain and CSF [8–10, 60–63]. Mounting evidence supports the existence of calcium dyshomeostasis and its consequent activation of calpain and caspases in aging cells/brain and age-related neurodegenerative diseases including AD, PD, and HD. Early and emerging new data point to abnormality of spectrin degradation and accumulation of SBDPs in the brains from AD patients and transgenic animal models [13, 86••]. Accordingly, we propose that SBDPs may serve as novel biomarkers for chronic neurodegenerative diseases such as AD, and solicit further exploration toward this direction.
Developing and validating specific antibodies for detection of SBDPs are the crucial first steps for such an effort. For instance, antibodies that selectively detect SBDPs but not the hiding epitope(s) in the uncleaved spectrin proteins would be of particular importance in characterization of SBDP accumulation in the brain and CSF in anatomical and biochemical assays. Antibodies that may specifically recognize brain or neuronal spectrin cleavage products would be of advantage by allowing analysis of the products in CSF and perhaps in blood. Antibodies that preferentially recognize calpain- and caspase-specific fragments may help understand the sequential or orderly processing of spectrin cleavages executed by these enzymes anatomically and biochemically in the brain and in model cell systems. Anatomically usable specific SBDP antibodies are essential for profiling the spatiotemporal course of physiological versus pathophysiological spectrin cleavage and accumulation of SBDPs relative to cellular integrity or pathogenic progression.
As discussed earlier, calcium-dependent calpain and caspase activation and resultant spectrin proteolysis and SBDP elevation appear to represent a general event in acute and chronic neurodegenerative conditions. Therefore, sensitivity and specificity are issues of concern in regard to the application of SBDPs as biomarkers for neurodegenerative diseases. Conceivably, a biomarker or diagnostic tool needs to be used adjunctively, in combination with other laboratory measurements, clinical history, and symptom presentations. Obviously, basic and translational investigations are required to further determine whether SBDPs are useful for assessing a certain aspect of neuropathology in age-related neurodegenerative diseases. Currently, numerous animal models of these diseases are available for initial studies of SBDPs in the brain and CSF. For instance, anatomical and biochemical examination of SBDP accumulation in the brain of transgenic AD model rodents may reveal data about the extent, localization, and time course of SBDP alterations relative to development of amyloid plaques, tauopathy, and neuronal death. Such comparative animal studies may provide information for further investigation into the role and position of aberrant proteolysis and SBDP accumulation in the human brain during aging and in AD.
Conclusions
As an abundant membrane protein, spectrin is cleaved by proteases including calpain and caspase-3 in a calcium-dependent manner to produce SBDPs. In the central nervous system, this modulation plays an important physiological role in neuroplasticity under normal conditions. However, this signal pathway is persistently activated under cellular stress, during aging, and in a variety of neurodegenerative diseases. Currently, SBDPs are being evaluated as novel biomarkers for traumatic and ischemic brain injury. It is worthy to explore whether these biomarkers also may be useful for detection/assessment of neuronal injury in the brain in chronic neurodegenerative diseases such as AD. This idea can be approached by carrying out additional basic and translational studies using existing animal models for human neurodegenerative diseases.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Marchesi VT, Steers Jr E. Selective solubilization of a protein component of the red cell membrane. Science. 1968;159:203–4.
Goodman SR, Zagon IS, Whitfield CF, et al. A spectrin-like protein from mouse brain membranes: immunological and structural correlations with erythrocyte spectrin. Cell Motil. 1983;3:635–47.
Bennett V, Lambert S. The spectrin skeleton: from red cells to brain. J Clin Invest. 1991;87:1483–9.
Baines AJ. Evolution of spectrin function in cytoskeletal and membrane networks. Biochem Soc Trans. 2009;37:796–803.
Ikeda Y, Dick KA, Weatherspoon MR, et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38:184–90.
Lynch G, Baudry M. Brain spectrin, calpain and long-term changes in synaptic efficacy. Brain Res Bull. 1987;18:809–15.
Czogalla A, Sikorski AF. Spectrin and calpain: a 'target' and a 'sniper' in the pathology of neuronal cells. Cell Mol Life Sci. 2005;62:1913–24.
Pineda JA, Lewis SB, Valadka AB, et al. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J Neurotrauma. 2007;24:354–66.
Weiss ES, Wang KK, Allen JG, et al. Alpha II-spectrin breakdown products serve as novel markers of brain injury severity in a canine model of hypothermic circulatory arrest. Ann Thorac Surg. 2009;88:543–50.
Zhang Z, Larner SF, Liu MC, et al. Multiple alphaII-spectrin breakdown products distinguish calpain and caspase dominated necrotic and apoptotic cell death pathways. Apoptosis. 2009;14:1289–98.
Mondello S, Robicsek SA, Gabrielli A, et al. αII-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients. J Neurotrauma. 2010;27:1203–13.
Peterson C, Vanderklish P, Seubert P, et al. Increased spectrin proteolysis in fibroblasts from aged and Alzheimer donors. Neurosci Lett. 1991;121:239–43.
Masliah E, Iimoto DS, Saitoh T, et al. Increased immunoreactivity of brain spectrin in Alzheimer disease: a marker for synapse loss? Brain Res. 1990;531:36–44.
Mangeat PH. Interaction of biological membranes with the cytoskeletal framework of living cells. Biol Cell. 1988;64:261–81.
Palek J, Lambert S. Genetics of the red cell membrane skeleton. Semin Hematol. 1990;27:290–332.
Winkelmann JC, Forget BG. Erythroid and nonerythroid spectrins. Blood. 1993;81:3173–85.
Delaunay J. The molecular basis of hereditary red cell membrane disorders. Blood Rev. 2007;21:1–20.
Gallagher PG. Update on the clinical spectrum and genetics of red blood cell membrane disorders. Curr Hematol Rep. 2004;3:85–91.
Karinch AM, Zimmer WE, Goodman SR. The identification and sequence of the actin-binding domain of human red blood cell beta-spectrin. J Biol Chem. 1990;265:11833–40.
Fowler V, Taylor DL. Spectrin plus band 4.1 cross-link actin. Regulation by micromolar calcium. J Cell Biol. 1980;85:361–76.
Davis JQ, Bennett V. Brain ankyrin. Purification of a 72,000 Mr spectrin-binding domain. J Biol Chem. 1984;259:1874–81.
Coleman TR, Harris AS, Mische SM, et al. Beta spectrin bestows protein 4.1 sensitivity on spectrin-actin interactions. J Cell Biol. 1987;104:519–26.
Fowler VM, Bennett V. Erythrocyte membrane tropomyosin. Purification and properties. J Biol Chem. 1984;259:5978–89.
Fowler VM. Tropomodulin: a cytoskeletal protein that binds to the end of erythrocyte tropomyosin and inhibits tropomyosin binding to actin. J Cell Biol. 1990;111:471–81.
Gardner K, Bennett V. Modulation of spectrin-actin assembly by erythrocyte adducin. Nature. 1987;328:359–62.
Morris CE. Mechanoprotection of the plasma membrane in neurons and other non-erythroid cells by the spectrin-based membrane skeleton. Cell Mol Biol Lett. 2001;6:703–20.
Mastrangelo M, Leuzzi V. Genes of early-onset epileptic encephalopathies: from genotype to phenotype. Pediatr Neurol. 2012;46:24–31.
Fukushima Y, Byers MG, Watkins PC, et al. Assignment of the gene for beta-spectrin (SPTB) to chromosome 14q23----q24.2 by in situ hybridization. Cytogenet Cell Genet. 1990;53:232–3.
Dhermy D, Galand C, Bournier O, et al. Hereditary spherocytosis with spectrin deficiency related to null mutations of the beta-spectrin gene. Blood Cells Mol Dis. 1998;24:251–61.
Gallagher PG, Petruzzi MJ, Weed SA, et al. Mutation of a highly conserved residue of betaI spectrin associated with fatal and near-fatal neonatal hemolytic anemia. J Clin Invest. 1997;99:267–77.
Stankewich MC, Tse WT, Peters LL, et al. A widely expressed betaIII spectrin associated with Golgi and cytoplasmic vesicles. Proc Natl Acad Sci U S A. 1998;95:14158–63.
Berghs S, Aggujaro D, Dirkx Jr R, et al. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002.
Stabach PR, Morrow JS. Identification and characterization of beta V spectrin, a mammalian ortholog of Drosophila beta H spectrin. J Biol Chem. 2000;275:21385–95.
Jiang X, Gillen S, Esposito I, et al. Reduced expression of the membrane skeleton protein beta1-spectrin (SPTBN1) is associated with worsened prognosis in pancreatic cancer. Histol Histopathol. 2010;25:1497–506.
Bennett V, Davis J, Fowler WE. Brain spectrin, a membrane-associated protein related in structure and function to erythrocyte spectrin. Nature. 1982;299:126–31.
Bennett V, Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol Med. 2008;4:28–36.
Goodman SR, Zimmer WE, Clark MB, et al. Brain spectrin: of mice and men. Brain Res Bull. 1995;36:593–606.
Zagon IS, Higbee R, Riederer BM, Goodman SR. Spectrin subtypes in mammalian brain: an immunoelectron microscopic study. J Neurosci. 1986;6:2977–86.
Susuki K, Raphael AR, Ogawa Y, et al. Schwann cell spectrins modulate peripheral nerve myelination. Proc Natl Acad Sci U S A. 2011;108:8009–80014.
Koenig E, Kinsman S, Repasky E, Sultz L. Rapid mobility of motile varicosities and inclusions containing alpha-spectrin, actin, and calmodulin in regenerating axons in vitro. J Neurosci. 1985;5:715–29.
Bloch RJ, Morrow JS. An unusual beta-spectrin associated with clustered acetylcholine receptors. J Cell Biol. 1989;8:481–93.
Sunderland WJ, Son YJ, Miner JH, et al. The presynaptic calcium channel is part of a transmembrane complex linking a synaptic laminin (alpha4beta2gamma1) with non-erythroid spectrin. J Neurosci. 2000;20:1009–19.
Lacas-Gervais S, Guo J, Strenzke N, et al. BetaIVSigma1 spectrin stabilizes the nodes of Ranvier and axon initial segments. J Cell Biol. 2004;166:983–90.
Hülsmeier J, Pielage J, Rickert C, et al. Distinct functions of alpha-spectrin and beta-spectrin during axonal pathfinding. Development. 2007;134:713–22.
Yang Y, Ogawa Y, Hedstrom KL, Rasband MN. betaIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrin G. J Cell Biol. 2007;176:509–19.
Voas MG, Lyons DA, Naylor SG, et al. alphaII-spectrin is essential for assembly of the nodes of Ranvier in myelinated axons. Curr Biol. 2007;7:562–8.
Puchkov D. Leshchyns'ka I, Nikonenko AG, et al.: NCAM/spectrin complex disassembly results in PSD perforation and postsynaptic endocytic zone formation. Cereb Cortex. 2011;21:2217–32.
Pielage J, Fetter RD, Davis GW. A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J Cell Biol. 2006;175:491–503.
Ramser EM, Buck F, Schachner M, Tilling T. Binding of alphaII spectrin to 14-3-3beta is involved in NCAM-dependent neurite outgrowth. Mol Cell Neurosci. 2010;45:66–74.
Stankewich MC, Gwynn B, Ardito T, et al. Targeted deletion of betaIII spectrin impairs synaptogenesis and generates ataxic and seizure phenotypes. Proc Natl Acad Sci U S A. 2010;107:6022–7.
Westphal D, Sytnyk V, Schachner M. Leshchyns'ka I: Clustering of the neural cell adhesion molecule (NCAM) at the neuronal cell surface induces caspase-8- and -3-dependent changes of the spectrin meshwork required for NCAM-mediated neurite outgrowth. J Biol Chem. 2010;285:42046–57.
Gao Y, Perkins EM, Clarkson YL, et al. β-III spectrin is critical for development of Purkinje cell dendritic tree and spine morphogenesis. J Neurosci. 2011;31:16581–90.
Nestor MW, Cai X, Stone MR, et al. The actin binding domain of βI-spectrin regulates the morphological and functional dynamics of dendritic spines. PLoS. 2011;6:e16197.
Lorenzo DN, Li MG, Mische SE, et al. Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila. J Cell Biol. 2010;189:143–58.
Perkins EM, Clarkson YL, Sabatier N, et al. Loss of beta-III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of spinocerebellar ataxia type 5 in humans. J Neurosci. 2010;30:4857–67.
Nath R, Raser KJ, Stafford D, et al. Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319:683–90.
Hsu YJ, Zimmer WE, Goodman SR. Erythrocyte spectrin’s chimeric E2/E3 ubiquitin conjugating/ligating activity. Cell Mol Biol (Noisy-le-grand). 2005;51:187–93.
Wang KK, Posmantur R, Nath R, et al. Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem. 1998;273:22490–7.
Zhao X, Newcomb JK, Pike BR, et al. Novel characteristics of glutamate-induced cell death in primary septohippocampal cultures: relationship to calpain and caspase-3 protease activation. J Cereb Blood Flow Metab. 2000;20:550–62.
Newcomb-Fernandez JK, Zhao X, Pike BR, et al. Concurrent assessment of calpain and caspase-3 activation after oxygen-glucose deprivation in primary septo-hippocampal cultures. J Cereb Blood Flow Metab. 2001;21:1281–94.
Pike BR, Flint J, Dave JR, et al. Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived alphaII-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2004;24:98–106.
Gold MS, Kobeissy FH, Wang KK, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66:118–27.
Vanderklish PW, Bahr BA. The pathogenic activation of calpain: a marker and mediator of cellular toxicity and disease states. Int J Exp Pathol. 2000;81:323–239.
Bahr BA, Tiriveedhi S, Park GY, Lynch G. Induction of calpain-mediated spectrin fragments by pathogenic treatments in long-term hippocampal slices. J Pharmacol Exp Ther. 1995;273:902–8.
Seubert P, Larson J, Oliver M, et al. Stimulation of NMDA receptors induces proteolysis of spectrin in hippocampus. Brain Res. 1988;460:189–94.
Siman R, Noszek JC, Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J Neurosci. 1989;9:1579–90.
Bi X, Chang V, Siman R, et al. Regional distribution and time-course of calpain activation following kainate-induced seizure activity in adult rat brain. Brain Res. 1996;726:98–108.
Tamada Y, Nakajima E, Nakajima T, et al. Proteolysis of neuronal cytoskeletal proteins by calpain contributes to rat retinal cell death induced by hypoxia. Brain Res. 2005;1050:148–55.
Huang W, Fileta J, Rawe I, Qu J, Grosskreutz CL. Calpain activation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:3049–54.
Hwang IK, Yoo KY, Kim DW, et al. AlphaII-spectrin breakdown product increases in principal cells in the gerbil main olfactory bulb following transient ischemia. Neurosci Lett. 2008;435:251–6.
Farkas O, Polgár B, Szekeres-Barthó J, et al. Spectrin breakdown products in the cerebrospinal fluid in severe head injury–preliminary observations. Acta Neurochir (Wien). 2005;147:855–61.
Brophy GM, Pineda JA, Papa L, et al. alphaII-Spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. J Neurotrauma. 2009;26:471–9.
Mattson MP. Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal. 2006;8:1997–2006.
Raynaud F, Marcilhac A. Implication of calpain in neuronal apoptosis. A possible regulation of Alzheimer's disease. FEBS J. 2006;273:3437–43.
Levy OA, Malagelada C, Greene LA. Cell death pathways in Parkinson's disease: proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14:478–500.
Vicencio JM, Lavandero S, Szabadkai G. Ca2+, autophagy and protein degradation: thrown off balance in neurodegenerative disease. Cell Calcium. 2010;47:112–21.
Mouatt-Prigent A, Karlsson JO, Agid Y, Hirsch EC. Increased M-calpain expression in the mesencephalon of patients with Parkinson's disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience. 1996;73:979–87.
Alvira D, Ferrer I, Gutierrez-Cuesta J, et al. Activation of the calpain/cdk5/p25 pathway in the girus cinguli in Parkinson's disease. Parkinsonism Relat Disord. 2008;14:309–13.
Dufty BM, Warner LR, Hou ST, et al. Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol. 2007;170:1725–38.
Gafni J, Ellerby LM. Calpain activation in Huntington's disease. J Neurosci. 2002;22:4842–9.
Kim YJ, Yi Y, Sapp E, et al. Caspase 3-cleaved N-terminal fragments of wild-type and mutant huntingtin are present in normal and Huntington's disease brains, associate with membranes, and undergo calpain-dependent proteolysis. Proc Natl Acad Sci U S A. 2001;98:12784–9.
Wellington CL, Ellerby LM, Gutekunst CA, et al. Caspase cleavage of mutant huntingtin precedes neurodegeneration in Huntington's disease. J Neurosci. 2002;22:7862–72.
Gafni J, Hermel E, Young JE, et al. Inhibition of calpain cleavage of huntingtin reduces toxicity: accumulation of calpain/caspase fragments in the nucleus. J Biol Chem. 2004;279:20211–20.
Nilsson E, Alafuzoff I, Blennow K, et al. Calpain and calpastatin in normal and Alzheimer-degenerated human brain tissue. Neurobiol Aging. 1990;11:425–31.
Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A. 1993;90:2628–32.
•• Higuchi M, Iwata N, Matsuba Y, et al. Mechanistic involvement of the calpain-calpastatin system in Alzheimer neuropathology. FASEB J. 2011; doi:10.1096/fj.11-187740. This study reports that cerebrospinal fluid from patients with AD contained a higher level of calpain-cleaved spectrin than that of control patients.
Rohn TT, Head E. Caspase activation in Alzheimer's disease: early to rise and late to bed. Rev Neurosci. 2008;19:383–93.
Garg S, Timm T, Mandelkow EM, et al. Cleavage of Tau by calpain in Alzheimer's disease: the quest for the toxic 17 kD fragment. Neurobiol Aging. 2011;32:1–14.
Gaczyńska M. Changes in proteolytic susceptibility of human erythrocyte membrane proteins during red blood cell aging. Cytobios. 1992;72:197–200.
Lee A, Morrow JS, Fowler VM. Caspase remodeling of the spectrin membrane skeleton during lens development and aging. J Biol Chem. 2001;276:20735–42.
Bahr BA, Vanderklish PW, Ha LT, et al. Spectrin breakdown products increase with age in telencephalon of mouse brain. Neurosci Lett. 1991;131:237–40.
Bernath E, Kupina N, Liu MC, et al. Elevation of cytoskeletal protein breakdown in aged Wistar rat brain. Neurobiol Aging. 2006;27:624–32.
Ayala-Grosso C, Tam J, Roy S, et al. Caspase-3 cleaved spectrin colocalizes with neurofilament-immunoreactive neurons in Alzheimer's disease. Neuroscience. 2006;141:863–74.
•• Liang B, Duan BY, Zhou XP, et al. Calpain activation promotes BACE1 expression, amyloid precursor protein processing, and amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2010;285:27737–44. This study shows elevation of calpain-mediated SBDPs in the brain in a transgenic model of AD, and attenuation of amyloid plaque pathogenesis and tau phosphorylation by in vivo inhibition of calpain activity.
Zhang XM, Cai Y, Xiong K, et al. Beta-secretase-1 elevation in transgenic mouse models of Alzheimer's disease is associated with synaptic/axonal pathology and amyloidogenesis: implications for neuritic plaque development. Eur J Neurosci. 2009;30:2271–83.
Acknowledgements
The authors thank Drs. Yan Cai and Xie-Mei Zhang for excellent technical assistance, and the National Natural Science Foundation of China (grant #81171091) for supporting Alzheimer’s disease research in our laboratory.
Disclosures
X.-X. Yan: none. Dr. Andreas Jeromin is employed by Banyan Biomarkers, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, XX., Jeromin, A. Spectrin Breakdown Products (SBDPs) as Potential Biomarkers for Neurodegenerative Diseases. Curr Tran Geriatr Gerontol Rep 1, 85–93 (2012). https://doi.org/10.1007/s13670-012-0009-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13670-012-0009-2